JOURNAL 3664
Journal of Chemical Metrology
Available Online: November 22,2025
p.1 - 8
http://doi.org/10.25135/jcm.123.2510.3664 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 66 times.
GRAPHICAL ABSTRACT
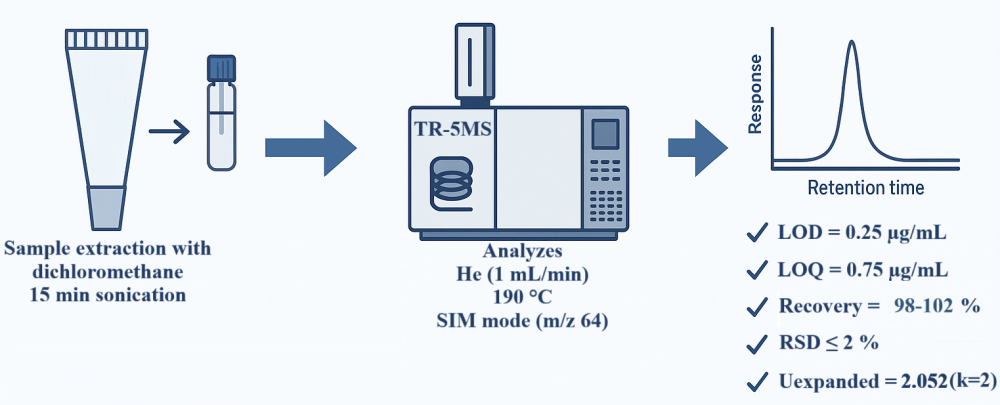
ABSTRACT
A simple, sensitive, and validated gas chromatography–mass spectrometry (GC–MS) method was developed for the quantitative determination of sulfur in topical formulations. The separation was achieved on a TR-5MS capillary column (30 m × 0.25 mm ID, 0.25 µm film thickness) using helium as the carrier gas at a flow rate of 1 mL/min. The oven temperature was maintained at 190 °C, and the total analysis time was 13 min. Detection was performed in selective ion monitoring (SIM) mode at m/z 64. The injection volume was 1 µL. The method exhibited excellent linearity within the concentration range of 0.75–15.00 µg/mL. The lower limit of detection (LOD) and quantification (LOQ) were found to be 0.25 µg/mL and 0.75 µg/mL, respectively. Mean recovery values ranged from 98% to 102%, with intra- and inter-day precisions (RSD) not exceeding 2%. Method validation was conducted in accordance with the ICH Q2(R1) guidelines. Furthermore, measurement uncertainty was evaluated following the EURACHEM approach. The validated method was successfully applied to the determination of sulfur in commercial topical formulations, demonstrating its suitability for routine quality control analysis.
KEYWORDS- Sulfur
- topical formulations
- method validation
- GC-MS
- uncertainty assessment.
SUPPORTING INFORMATION
Supporting Files
Supporting Files
Download File Supporting Files.docx (80.32 KB)