JOURNAL 1451
Bioorganic and Medicinal Chemistry Reports
Year: 2019 Issue: 1-2 (January-December)
p.15 - 23
Viewed 3894 times.
GRAPHICAL ABSTRACT
ABSTRACT
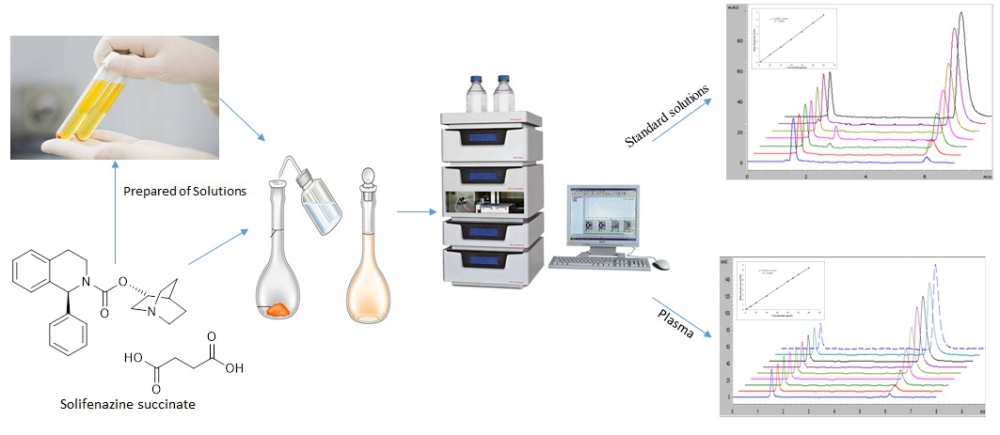
An accurate, rapid and precise high performance liquid chromatographic coupled with ultraviolet detector method has been developed for the determination of solifenazine succinate in pharmaceutical preparation and human plasma. Quantitative analysis of solifenazine succinate was performed using a C18 column at wavelength at 210 nm. The mobile phase consisted of 10 mM ammonium formate buffer (pH 4.0), acetonitrile and methanol (52.5:32.5:12.5 v/v/v). The method showed linearity in the concentration range of 3-60 μg/mL and the correlation coefficient was 0.9998 and 0.9994 for the standard and human plasma solutions, respectively. The proposed method was within acceptable limits in terms of accuracy, precision, selectivity, linearity, sensitivity and recovery parameters. This method is readily applicable for the determination of solifenazine succinate in human plasma and pharmaceutical preparations.
KEYWORDS- Solifenacin succinate
- pharmaceutical formulations
- human plasma
- HPLC