JOURNAL 1706
Bioorganic and Medicinal Chemistry Reports
Year: 2020 Issue: 2 July-December
p.22 - 31
Viewed 2784 times.
GRAPHICAL ABSTRACT
ABSTRACT
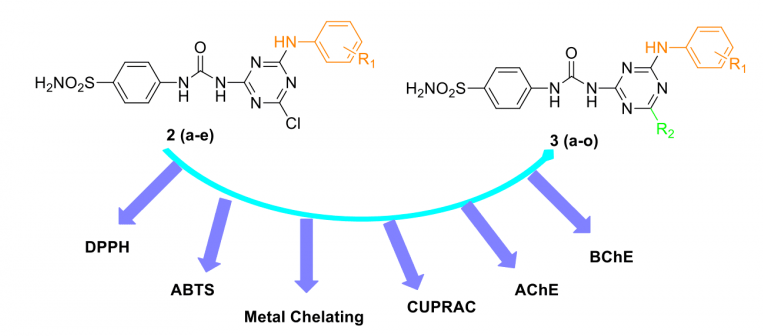
A series of twenty 1,3,5-triazine-substituted ureido benzenesulfonamides 2 (a-e) and 3 (a-o) were re-synthesized and assayed for antioxidant properties by using several different methods including 1,1-diphenyl-2-picryl hydrazyl (DPPH) free radical scavenging assay, 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) cation radical decolarization assay, metal chelating and cupric reducing antioxidant capacity (CUPRAC) methods. The inhibitory effects of compounds on acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes have been also demonstrated. All compounds showed a greater antioxidant capacity against ABTS assay by having a more potent activity than the standards BHT, BHA and α-TOC. In general, all compounds were non susceptible to against AChE enzyme. On the other hand, several lead compounds were obtained from the current series against BChE enzyme. More specifically, compound 3m showed great inhibition profile against BChE with % inhibition value of 93.77, which is better than the standard drug galantamine (% inhibition value of 87.86).
KEYWORDS- 1,3,5-triazine; sulfonamide
- acetylcholinesterase
- butyrylcholinesterase
- antioxidant