JOURNAL 1349
Journal of Chemical Metrology
Year: 2019 Issue: 2 July-December
p.80 - 83
Viewed 3795 times.
GRAPHICAL ABSTRACT
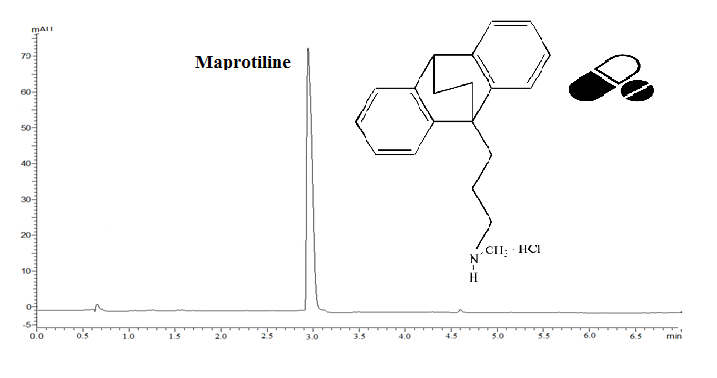
ABSTRACT
This current study aimed to develop a simple, fast, and reproducible isocratic reverse-phase ultra-fast liquid chromatographic (RP-UFLC) method to detect and quantify maprotiline hydrochloride (MAP) in bulk drug and pharmaceutical formulations. Chromatographic separation was accomplished on a C18 column (100 × 4mm, 3 μm) under isocratic elution with the use of a binary solution of acetonitrile and phosphate buffer at a pH of 7 (75 : 25, v/v) and a flow rate of 0.4 mL per minute at 215 nm. The linearity was excellent in the concentration range of MAP from 0.1 to 1.5 µg/mL with a regression coefficient of 0.9996. The proposed method was validated with the respective ICH guidelines. The drug was subjected to hydrolytic, acidic, basic, thermal, photolytic, and oxidative stress conditions as required by the ICH regulation. The method was found to be suitable for use in routine practice to analyze MAP in the pharmaceutical dosage form.
KEYWORDS- Maprotiline,
- UFLC
- method alidation
- stability Indicating
- tablets
- quality control analysis