JOURNAL 1518
Journal of Chemical Metrology
Year: 2020 Issue: 1 January-June
p.35 - 41
Viewed 3254 times.
GRAPHICAL ABSTRACT
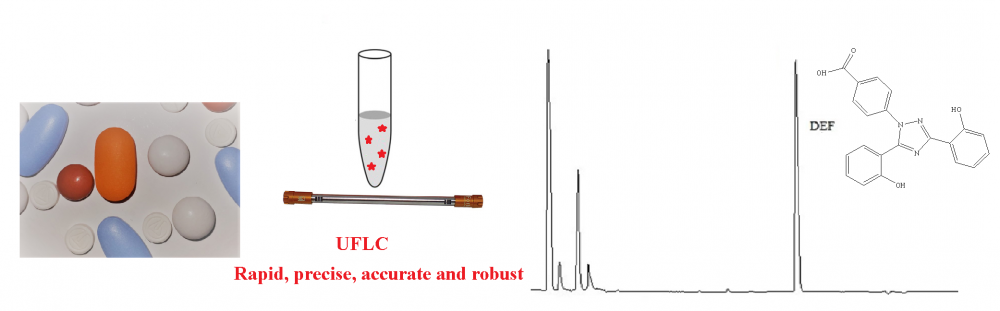
ABSTRACT
In the current study, a new sensitive and precise method based on HPLC and derivatization with dansyl chloride was developed for quantification of deferasirox (DEF) in pharmaceutical dosage forms and spiked plasma samples. Separation procedure in the chromatographic system was performed using a mobile phase consisting of the mixture of methanol and acetic acid solution (0.5 M, pH adjusted to 7.0 with NaOH) with a ratio of 70:30 v/v at flow rate of 1.0 mL per minute under isocratic elution on a C18 column (150 mm × 4.6 mm, 5 μm I.D.). The excitation wavelength was selected as 340 nm and emission wavelength was selected as 480 nm for the measurement of the analyte signal. The retention time for DEF is approximately 3.5 min. ICH Guidelines were taken into account for method validation. For the deferasirox concentration range of 20–2000 ng/mL, the proposed analytical method exhibited a linear relationship between the drug concentration and measured fluorescence with a correlation coefficient of 0.9994.The currently developed method can be implemented efficiently for the quantification of DEF in pharmaceutical dosage forms and spiked plasma samples.
KEYWORDS- Deferasirox
- HPLC
- method validation
- pharmaceutical formulation
- spiked plasma