JOURNAL 1622
Journal of Chemical Metrology
Year: 2020 Issue: 1 January-June
p.69 - 76
Viewed 3513 times.
GRAPHICAL ABSTRACT
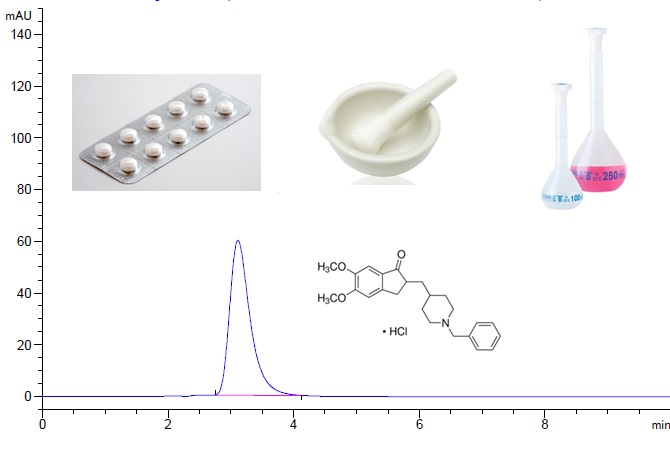
ABSTRACT
Donepezil HCl is a hydrochloride salt of a piperidine derivative acetylcholinesterase inhibitor and, it uses in treatment demantia of Alzheimer’s disease. In this study, a sensitive and rapid HPLC-UV method was developed and validated for determination of Donepezil HCl in API and tablet dosage forms. Chromatographic separation was performed using a Ace 5 C18 (5 μm, 250 x 4.6 mm) by using isocratic phosphate buffer at pH:2.0 and acetonitrile (55:45, v/v) mobile phase was used at the rate of 1.2 mL/min. The column temperature was set at 30 °C and the UV detection was recorded at 268 nm. The method was validated with respect to specificity, precision, accuracy, linearity, repeatability and reproducibility parameters in a concentration range of 25-125 µg/mL. The limit of detection (LOD) and limit of quantification (LOQ) were determined as 1.40 and4.20 µg/mL, respectively. The uncertainty budget of the measurement for Donepezil HCl was estiamted as 5.80 % at 95% confidence level (k = 2).
KEYWORDS- Donepezil HCl
- HPLC-UV
- pharmaceuticals
- method validation
- measurement uncertainty