JOURNAL 1701
Journal of Chemical Metrology
Year: 2020 Issue: 2 July-December
p.153 - 159
Viewed 4821 times.
GRAPHICAL ABSTRACT
ABSTRACT
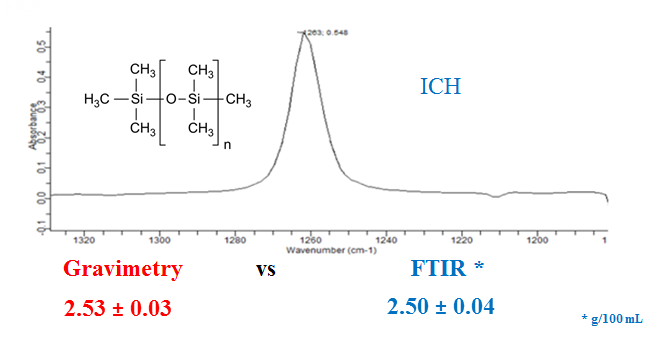
Fourier transform infrared (FTIR) spectroscopy was mostly used in both pharmacopeia and literature studies to determine the assay of simethicone (SMT) in pharmaceuticals. It should be noted that HPLC is used less frequently. As an alternative to the mentioned methods a new, simple, fast, easy-to-apply and very cheap gravimetric method was developed and validated according to ICH guidance entitled Q2B Validation of Analytical Procedures: Methodology for the quantification analysis of simethicone in different pharmaceutical forms. For the simethicone suspension product provided to alleviate too much gas in the gastrointestinal tract, the simethicone amount was determined both by the validated gravimetric method and by the FTIR method defined in the USP and BP monographs, and the results were within the acceptance criteria. It is emphasized that there is no significant difference between the results of gravimetric and FTIR methods according to the calculated F- and t-test results.
KEYWORDS- Simethicone
- gravimetry
- F- and t-test
- method validation
- assay