JOURNAL 2344
Journal of Chemical Metrology
Year: 2022 Issue: 1 January-June
p.38 - 48
Viewed 3252 times.
GRAPHICAL ABSTRACT
ABSTRACT
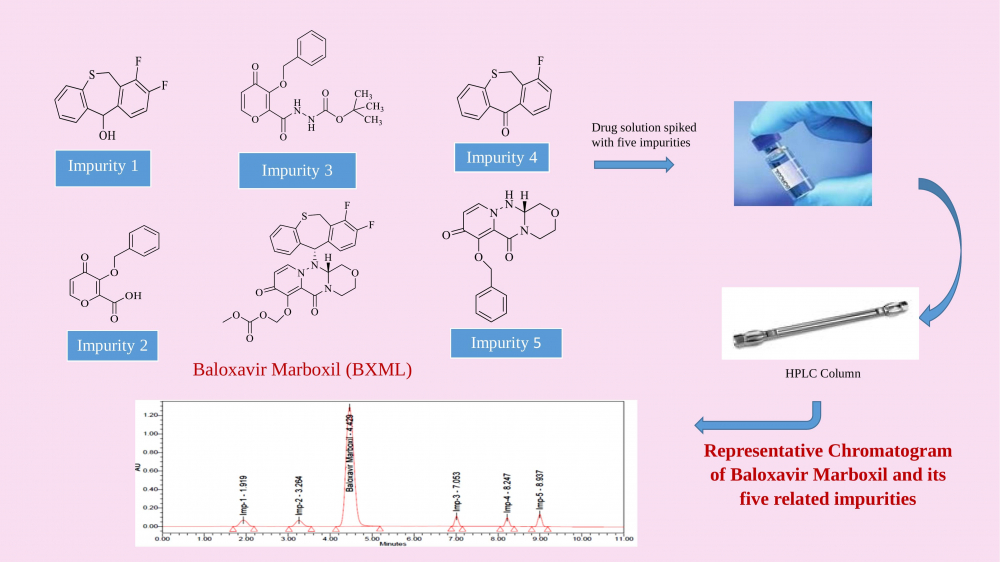
For the quantification of contaminants in Baloxavir marboxil (BXML), a simple and accurate RP-HPLC method was established and validated. BXML is an antiviral agent used to treat infections caused by influenza A and B viruses. To achieve optimal separation of all five impurities along with main moiety BXML the chromatography was carried out with X-Bridge Phenyl (150 x 4.6 mm) 3.5µ column with a mobile phase flow speed of 0.5 mL/minute. The mobile phase comprised of methanol and KH2PO4 buffer (pH 2.5) in gradient mode. The retention periods of Imp - 1, Imp - 2, Baloxavir marboxil, Imp - 3, Imp - 4 and Imp - 5 were found to be 1.919, 3.264, 4.429, 7.053, 8.247 and 8.937 min respectively. The developed method was found to be specific and linear (R2 > 0.999) for quantification of the target analytes. LOD was established as 0.020, 0.002, 0.001, 0.005, 0.002 and 0.002 µg/mL for BXML, Impurities 1, 2, 3, 4 and 5 respectively. Similarly, the LOQ was established as 0.066, 0.006, 0.003, 0.016, 0.006 and 0.006 µg/mL for Baloxavir marboxil, its Impurities 1,2,3,4 and 5 respectively. The % recovery by the assay was determined within the range of 98 –102%.
KEYWORDS- Baloxavir marboxil
- method development
- validation
- impurity
- RP-HPLC