JOURNAL 2859
Journal of Chemical Metrology
Year: 2023 Issue: 2 July-December
p.139 - 147
Viewed 2471 times.
GRAPHICAL ABSTRACT
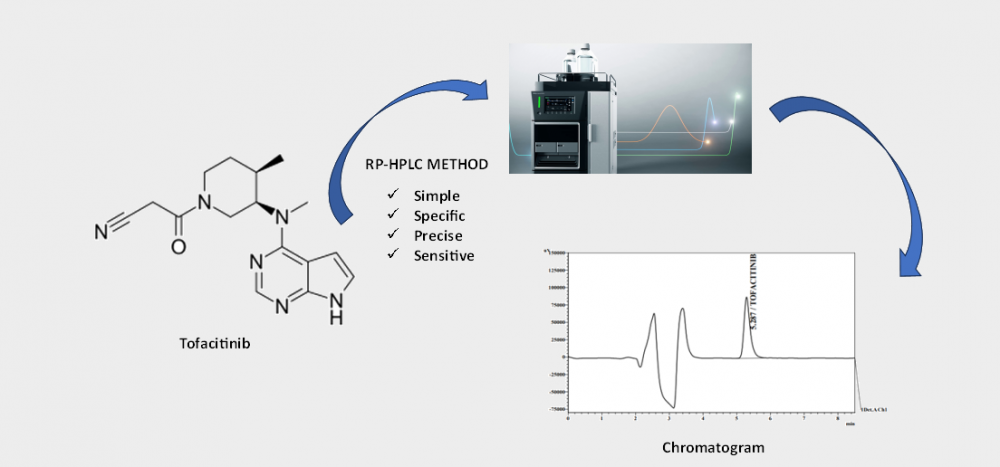
ABSTRACT
This study aimed to establish and validate a reliable RP-HPLC assay method for the quantification of tofacitinib (TFC), a janus kinase (JAK) inhibitor, in pharmaceutical formulations. The newly developed method exhibits simplicity, specificity, precision, and sensitivity. Experimental procedures utilized a Shimadzu Prominence 20A HPLC system equipped with a Inertsil ODS 3V C18 column (5µm particle size, 4.6 X 250 mm dimensions). The mobile phase, consisting of 0.05M ammonium acetate buffer at pH 5.0 and acetonitrile (65:35 v/v) in isocratic mode with a flow rate of 1.0 mL/min, facilitated accurate detection of the Tofacitinib peak at 230 nm wavelength. Comprehensive validation, including assessments of linearity, accuracy, precision, and robustness, was conducted in accordance with ICH requirements. The results demonstrated satisfaction, with a retention time (tR) of approximately 5.3 minutes. The imperative need for a swift and efficient RP-HPLC method for analyzing TFC led to the successful development and validation of this technique. Consequently, the RP-HPLC method has undergone thorough validation, establishing it as a user-friendly and trustworthy means for Tofacitinib analysis.
KEYWORDS- Tofacitinib
- RP-HPLC
- tablet
- validation
- assay method