JOURNAL 1039
Organic Communications
Year: 2018 Issue: 4 October-December
p.181 - 187
Viewed 4700 times.
GRAPHICAL ABSTRACT
ABSTRACT
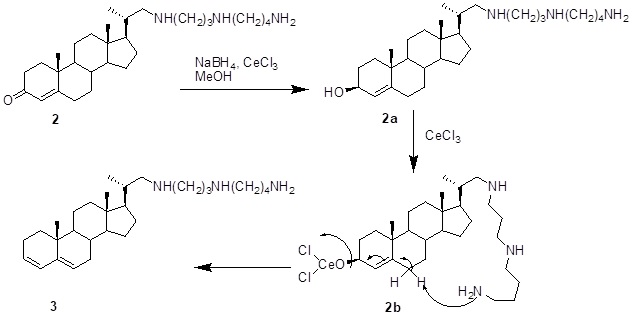
The conjugate reduction of a,b-unsaturated carbonyl compounds remains an active area of organic synthesis. Our aim is to control the reducing potential and selectivity of conjugated enone reduction. After much experimentation, the best conditions found for maximum yield with chemo and regioselectivity were to employ 1 molar eqiv. of NaBH4 for each mole of substrate in methanol containing some cerium(III) chloride. Many enones were converted essentially quantitatively to the allylic alcohol at room temperature. Surprisingly, reduction of the enone system in compound 2 (1.0 mmol) with NaBH4 (1.0mmol) and cerium chloride (1.0 mmol) in MeOH gave compound 3, in which the enone system was reduced and the allylic alcohol dehydrated producing the diene system.
KEYWORDS- Regioslective
- chemoselective
- reduction
- Luche reagent
- diene
- ketone