JOURNAL 1352
Organic Communications
VOLUME & ISSUE
Year: 2019 Issue: 3 July-September
Year: 2019 Issue: 3 July-September
PAGES
p.169 - 175
p.169 - 175
STATISTICS
Viewed 3384 times.
Viewed 3384 times.
AUTHORS
-
Oxana Kazakova

-
Liudmila Rubanik

-
Irina Smirnova

-
Olga Savinova

-
Anastasiya Petrova

-
Nikolay Poleschuk

-
Elmira Khusnutdinova

-
Eugene Boreko

-
Yuliya Kapustina

GRAPHICAL ABSTRACT
ABSTRACT
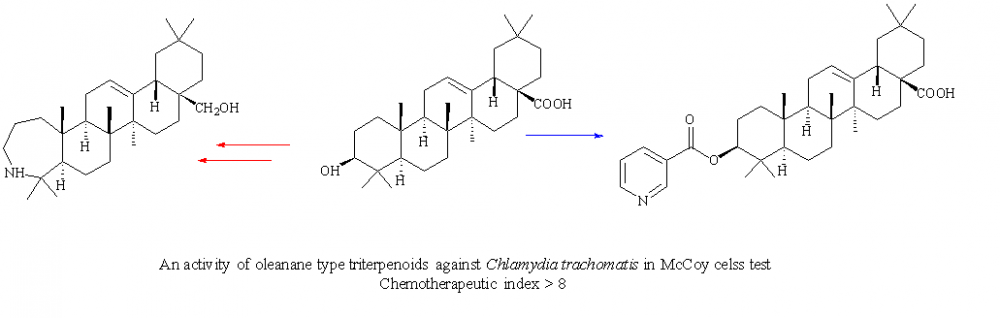
Modified synthesis of 3β-nicotinoyloxy-olean-12(13)-en-28-oic acid and 3-deoxy-3a-homo-3a-aza-28-hydroxy-olean-12(13)-en from natural occurring oleanolic acid is suggested. These compounds and two others of ursane and lupane type triterpenoids (3-oximino-urs-12-en-28-oic acid and 3-deoxy-3a-homo-3a-aza-28-hydroxy-lup-12(13)-en) were screened in vitro against Chlamydia trachomatis strain F-3271/Belarus/2015. Oleanane triterpenoids became the leading compounds with chemotherapeutic index > 8 and were chosen for further research.
KEYWORDS
- synthesis
- triterpenoids
- oleanane
- ursane
- Chlamydia trachomatis