JOURNAL 1765
Organic Communications
VOLUME & ISSUE
Year: 2020 Issue: 3 July-September
Year: 2020 Issue: 3 July-September
PAGES
p.79 - 88
p.79 - 88
STATISTICS
Viewed 2887 times.
Viewed 2887 times.
GRAPHICAL ABSTRACT
ABSTRACT
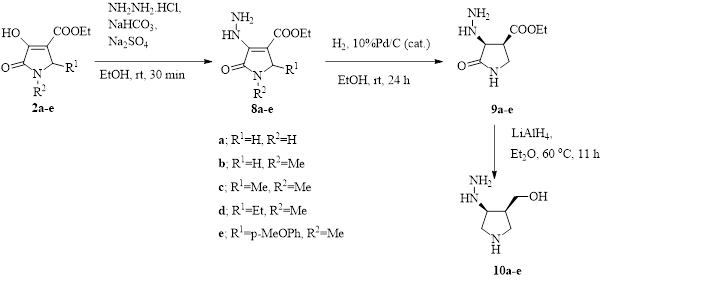
Some novel five-membered iminosugar analogues were efficiently synthesized via the MCRs of three components; amines, aldehydes and oxaloacetate to give 4-hydroxymethylpyrrolidines. Reduction of 3-hydroxy-4-carbomethoxy-3-pyrroliden-2-ones with Pd/C-catalyzed hydrogenation gave the corresponding cis-hydrogenated products at C3/C4 stereoselectively. Then following reductions of ester and amide functionalities with LiAlH4 afforded 4-(hydroxymethyl)pyrrolidin-3-ols. Among the synthesized compounds 4-hydrazineyl-3-hydroxymethyl-2-(4-methoxyphenyl)-N-methylpyrrolidine and 3-hydrazineyl-4-hydroxymethyl-1-methylpyrrolidine were found to be the most promising a-glucosidase inhibitor with IC50 values of 1.12 and 1.17 mM, respectively.
KEYWORDS- Iminosugar
- pyrrolidine
- one-pot reaction
- stereoselective hydrogenation
- antidiabetic