JOURNAL 2374
Organic Communications
Year: 2022 Issue: 3 July-September
p.273 - 287
Viewed 2334 times.
-
Oluwatoba Emmanuel Oyeneyin

-
Chiamaka G. Iwegbulam

-
Nureni Ipinloju

-
Bambo F. Olajide

-
Abel K. Oyebamiji

GRAPHICAL ABSTRACT
ABSTRACT
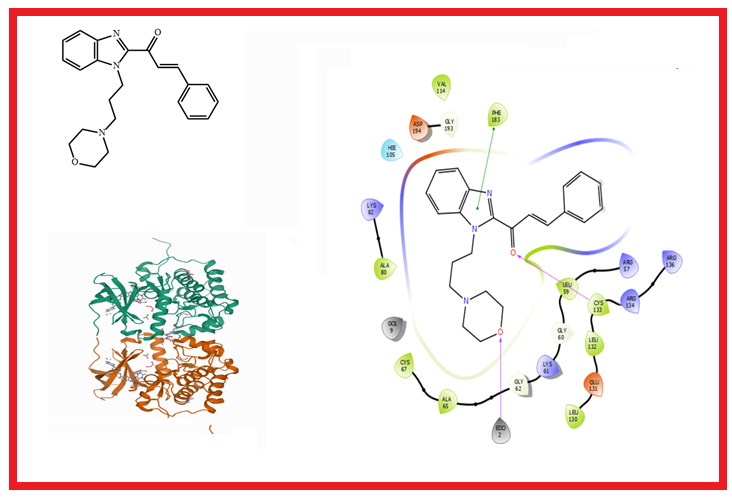
Cancer remains a threat to human existence owing the high number of deaths associated with it. Combating cancer has continued to garner interest from pharmaceutical companies as the resistance and toxicity issues have been observed with the treatment of known drugs. A series of benzimidazole-chalcone derivatives were obtained from literature and investigated for their ability to inhibit MCF-7 Breast Cancer Cell Lines via QSAR and molecular docking techniques. A QSAR model was generated to establish the relationship between molecular descriptors and bioactivity of benzimidazole-chalcone derivatives. The R2, adjusted R2, Q2 and R2pred values obtained suffice to deem the model fit and reliable. The binding affinities of the lead compounds were obtained via an extra precision docking procedure at the active site of the human serine/threonine-protein kinase receptor, 3FC2. Molecule 21 showed the closest binding affinity (-9.350 kcal/mol) as compared to doxorubicin (-9.305 kcal/mol), and had the best IC50 as compared to other compounds as reported earlier in literature. In-silico ADME and drug-likeness prediction of all the molecules showed good pharmacokinetic properties, bioavailability and non-toxicity. The model generated here could be used in predicting the bioactivity of novel antiproliferative agents.
KEYWORDS- Breast cancer
- MCF-7 cell line
- benzimidazoles
- quantitative structure activity relationship
- molecular docking
- pharmacokinetics