JOURNAL 3516
Organic Communications
VOLUME & ISSUE
Available Online: July 28,2025
Available Online: July 28,2025
PAGES
p.1 - 8
p.1 - 8
DOI ADDRESS
http://doi.org/10.25135/acg.oc.192.2505.3516 (DOI number will be activated after the manuscript has been available in an issue.)
http://doi.org/10.25135/acg.oc.192.2505.3516 (DOI number will be activated after the manuscript has been available in an issue.)
STATISTICS
Viewed 87 times.
Viewed 87 times.
GRAPHICAL ABSTRACT
ABSTRACT
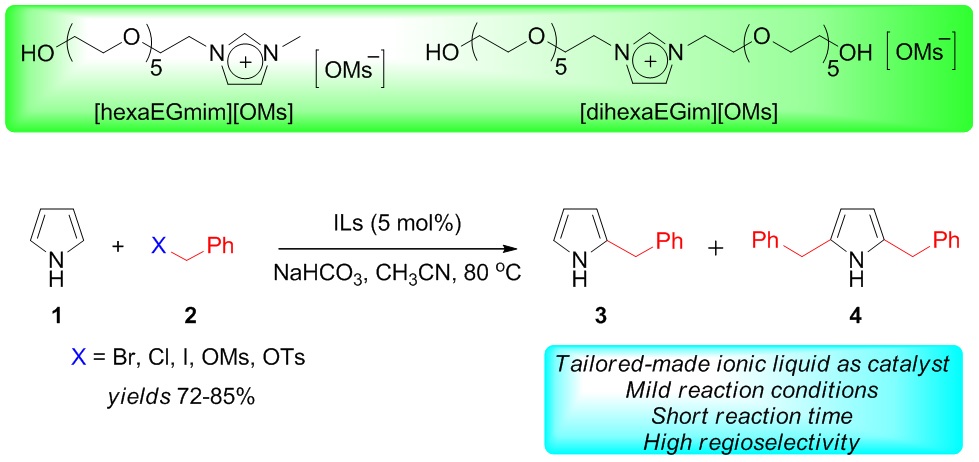
We have thoroughly investigated the C-benzylation of pyrrole (1) using different second and third generation ionic liquids (ILs). The pyrrole C-benzylation is achieved with benzyl halides, mesylate, and tosylate selectively at C2 position in good yield. Moreover, minimal byproducts under relatively mild conditions in hexaethylene glycol substituted imidazolium based ILs (hexaEGILs) have been observed. 2-Benzyl pyrrole (3) was synthesized in high yield from pyrrole (1) and benzyl bromide (2a) in the presence of [hexaEGmim][OMs] and [dihexaEGim][OMs] as two different tailor-made ILs as catalysts (10 mol%) in MeCN at 80 oC within an hour.
KEYWORDS- Pyrrole benzylation
- benzyl bromide
- ionic liquids
- ethylene glycols
- green chemistry