JOURNAL 2935
Records of Natural Products
Year: 2024 Issue: 1 January-February
p.165 - 170
Viewed 1369 times.
GRAPHICAL ABSTRACT
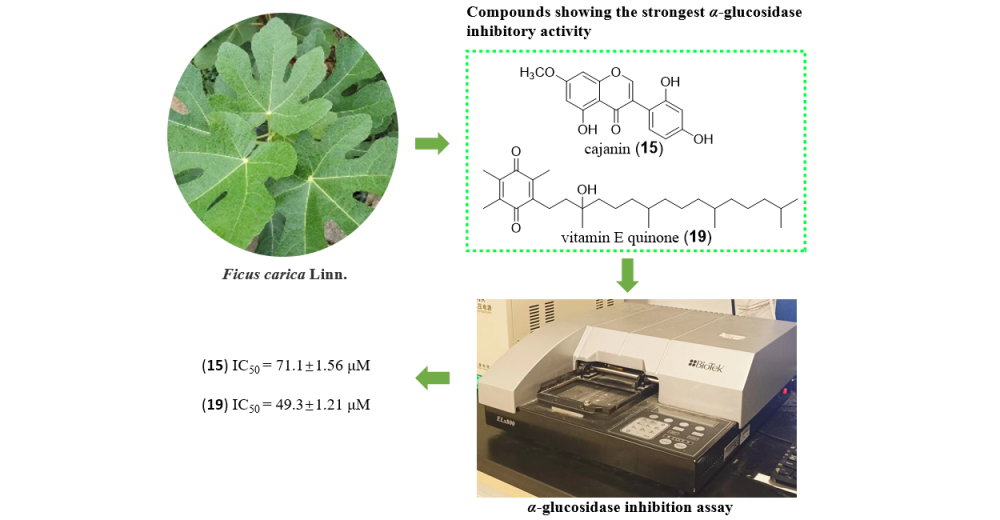
ABSTRACT
Nineteen compounds were purified from leaves of Ficus carica Linn. using various separation techniques such as recrystallization, column chromatography filled with silica gel, Sephadex LH-20, and MCI, and semi-preparative HPLC. By analysis of NMR, MS, and data comparison with those reported from literatures, their structures were identified as umbelliferone (1), psoralen (2), furopinnarin (3), 6,7-furano-hydrocoumaric acid (4), (E)-3-[5-(6-hydroxy) benzofuranyl] propenoic acid (5), (E)-3-(6-hydroxy-4-methoxy-5-benzofuranyl) propenoic acid (6), nodakenetin (7), oxypeucedanin hydrate (8), dihydrofurocoumarin (9), (E)-4-hydroxy-3,3,5-trimethy1-4-(3-oxobu-1-en-1-yl)-cyclohexan-1-one (10), dehydrovomifoliol (11), 4,5-dihydroblumenol A (12), blumenol A (13), 5-hydroxy-4′,7-dimethoxyisoflavone (14), cajanin (15), loliolide (16), indole-3-carboxaldehyde (17), 1H-indole-3-carboxylic acid (18), vitamin E quinone (19). Their α-glucosidase inhibitory activities were evaluated by IC50 values. Compounds 15 and 19 showed obvious α-glucosidase inhibitory activity with IC50 of 71.1±1.56 µM and 49.3 µM±1.21, respectively, and by the kinetics of enzymatic reaction, the inhibition type by which compound 15 acting against α-glucosidase was inferred as anticompetitive. Moreover, preliminary structure-activity relationship was recapticulated from the tested compounds.
KEYWORDS- Ficus carica Linn.
- coumarin
- α-glucosidase inhibitor
- enzyme kinetics