JOURNAL 3611
Records of Natural Products
Year: 2026 Issue: 1
p.5 - 5
Viewed 565 times.
GRAPHICAL ABSTRACT
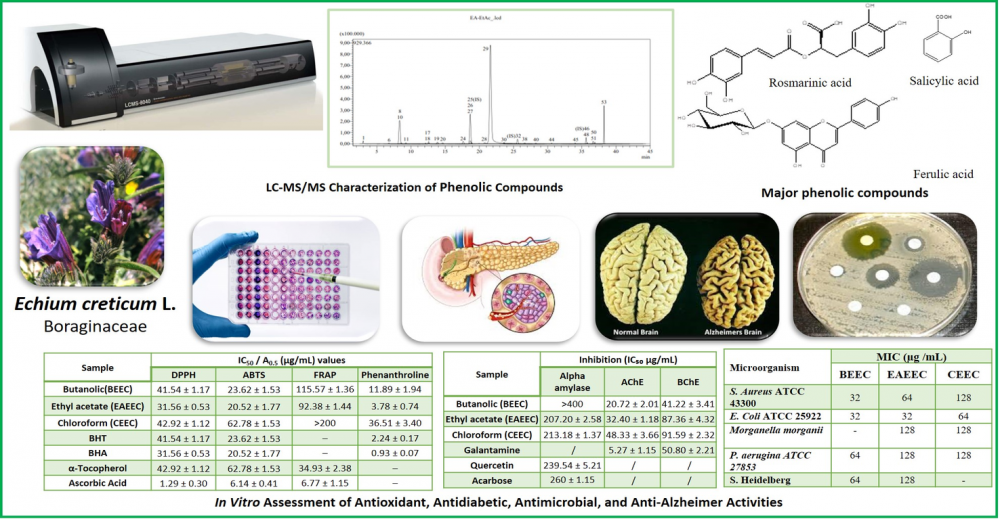
ABSTRACT
The present work provides the first comprehensive LC–MS/MS phytochemical profiling and pharmacological investigation of Echium creticum L., Boraginaceae, native to the Mediterranean region, used in folk medicine, The aerial parts were sequentially extracted using petroleum ether (PEEEC), chloroform (CEEC), ethyl acetate (EAEEC), and n-butanol (BEEC). LC-MS/MS profiling of the polar extracts identified 25, 22, and 24 bioactive compounds, with rosmarinic acid, ferulic acid, salicylic acid, and gentisic acid as major phenolics. Several flavonoid glycosides (cosmosiin, nicotiflorin, genistin) are reported for the first time in the Echium genus. Antioxidant activity was evaluated using DPPH, ABTS FRAP, and Fe³⁺-phenanthroline assays, was strongest in the ethyl acetate fraction. Antidiabetic potential was confirmed via α-amylase inhibition, where EAEEC demonstrated effective inhibition (IC₅₀ = 207.20 ± 2.58 µg/mL), comparable to quercetin and Acarbose. The antibacterial activity was also evaluated through the disc diffusion methods and MIC tests against a panel of 10 gram-positive (+) and gram-negative (-) bacterial strains (references and isolates multi-drug resistant bacteria) (MIC 32- 80 μg/mL). The n-butanol extract (BEEC) displayed the strongest anticholinesterase activity (IC₅₀ = 68.98 ± 1.33 µg/mL), consistent with its flavonoid glycoside enrichment. Altogether, these results highlight E. creticum as an underexplored source of phenolic compounds with multi-target in vitro bioactivities and expand the phytochemical diversity of the Echium genus.
KEYWORDS- Echium creticum
- LC–MS/MS
- phenolic compounds
- antioxidant
- anti-diabetic
- anticholinesterase
SUPPORTING INFORMATION
Ethical Exemption Statement
This document provides the official statement of exemption issued by the Head of the Bacteriology Department, Constantine University Hospital (CHU Ben-Badis, Algeria). It confirms that the anonymized clinical bacterial isolates used in this study were obtained as part of routine diagnostic activities, handled under institutional biosafety standards, and did not require formal ethical approval
Download File S2-Ethical exemption Statement.docx (4.54 MB)