JOURNAL 3250
Records of Natural Products
Available Online: September 03,2024
p.1 - 6
http://doi.org/10.25135/rnp.472.2406.3250 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 174 times.
-
Kongdech Savaspun

-
Chutamas Thepmalee

-
Hla Myo

-
Apiwan Arinno

-
Anuchit Phanumartwiwath

-
Chanat Aongbangkhen

-
Chatchakorn Eurtivong

-
Siriwat Boonchaisri

-
Padarat Ninjiaranai

-
Pornpat Sam-ang

GRAPHICAL ABSTRACT
ABSTRACT
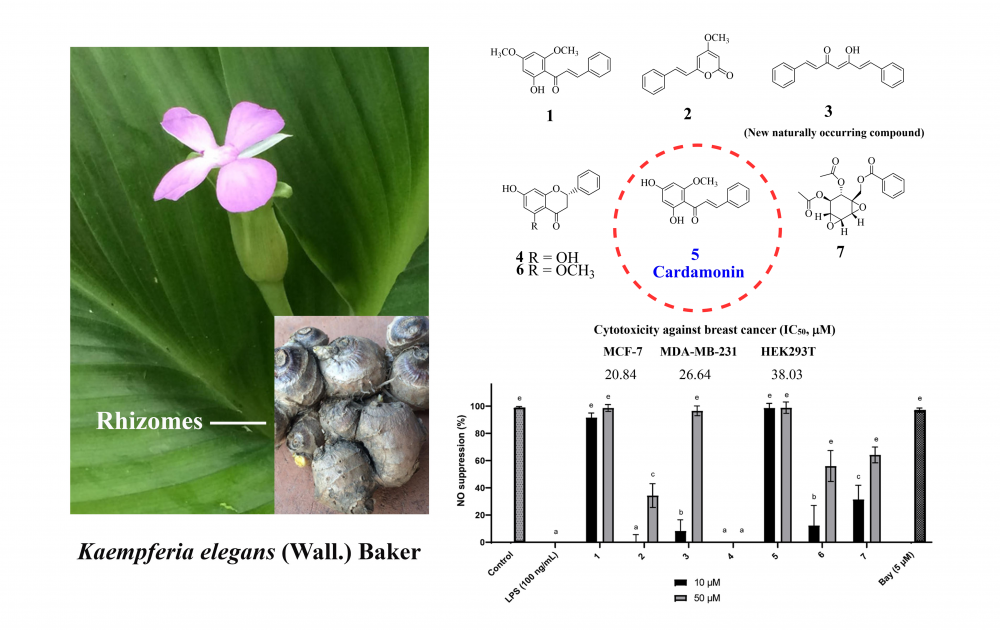
A new naturally occurring diarylheptanoid, (1E,4Z,6E)-5-hydroxy-1,7-diphenylhepta-1,4,6-trien-3-one (3), was isolated from the rhizomes of K. elegans along with six known compounds, flavokawain B (1), 5,6-dehydrokawain (2), pinocembrin (4), cardamonin (5), alpinetin (6), and crotepoxide (7), among which compound 6 had not previously been isolated from this plant species. Two chalcones, flavokawain B (1) and cardamonin (5) were active against nitric oxide (NO) radicals released from LPS-induced RAW264.7 macrophages, resulting in 91.58% and 98.68% inhibition of NO production, respectively. Furthermore, compounds 1 and 5 showed superior cytotoxicity against MCF-7 (IC50 = 23.07 and 20.84 mM) and MDA-MB-231 (IC50 = 21.77 and 26.64 mM) cell lines, respectively. In silico molecular modeling studies of the most active compounds 1 and 5 against epidermal growth factor receptors (EGFR) suggested that π–π interactions with residues on the EGFR protein contributed to their anticancer properties. The results suggest that cardamonin (5) could be a promising candidate for further development of anti-inflammatory and anticancer agents.
KEYWORDS- Kaempferia elegans
- flavokawain B
- cardamonin
- anti-inflammation
- human cancer cell
- molecular modeling