JOURNAL 3321
Records of Natural Products
Available Online: January 04,2025
p.1 - 6
http://doi.org/10.25135/rnp.491.2409.3321 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 439 times.
-
José L. Salvador-Hernández

-
Luis J. Calvillo-Carranza

-
Rosa E. del Río

-
Joel E. López-Meza

-
Alejandra Ochoa-Zarzosa

-
Julio C. Ontiveros-Rodríguez

-
Carlos M. Cerda-García-Rojas

-
Hugo A. García-Gutiérrez

GRAPHICAL ABSTRACT
ABSTRACT
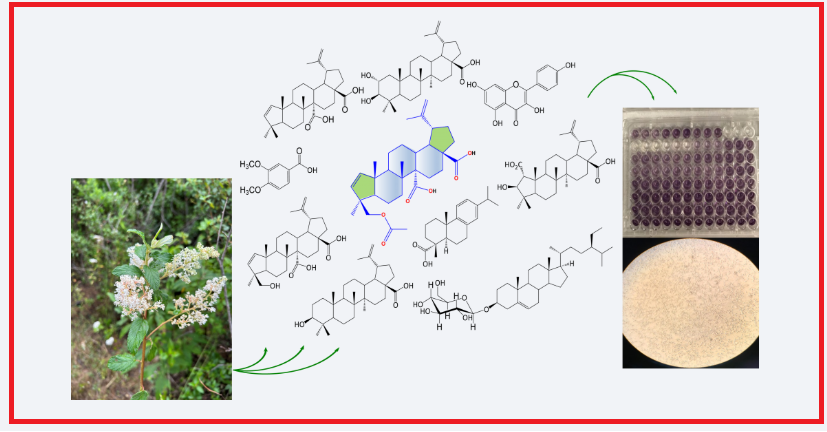
The dichloromethane (DCM) and ethyl acetate (EtOAc) extracts of C. caeruleus yielded nine known compounds, including an A-nor-lupane triterpenoid identified as gouanic acid B (1). Additionally, its acetyl derivative, acetylgouanic acid B (2), is reported here for the first time as a natural product. Furthermore, we tested the bioactivity of natural products 1 and 2 and their dimethyl ester derivatives 3 and 4 against cancer cell lines MCF-7, A549, HeLa, and K562. Among these, compounds 1 (IC50 = 36.4 ± 4.0 μM), 2 (IC50 = 21.6 ± 4.3 μM), and 4 (IC50 = 33.0 ± 2.0 μM) demonstrated moderate to good activity against the K562 cell line while maintaining a satisfactory survival rate in non-cancerous bMEC cells. Notably, the natural triterpenes 1 and 2 and derivative 4 showed remarkable outcomes in cytotoxicity tests due to their specificity against K562 leukemia cells.
KEYWORDS- Ceanothus caeruleus
- triterpenoid
- bioactive compounds
- cytotoxicity activity
- non-cancerous cell line