JOURNAL 1104
Bioorganic and Medicinal Chemistry Reports
VOLUME & ISSUE
Year: 2018 Issue: 1 January-December
Year: 2018 Issue: 1 January-December
PAGES
p.22 - 28
p.22 - 28
STATISTICS
Viewed 3879 times.
Viewed 3879 times.
GRAPHICAL ABSTRACT
ABSTRACT
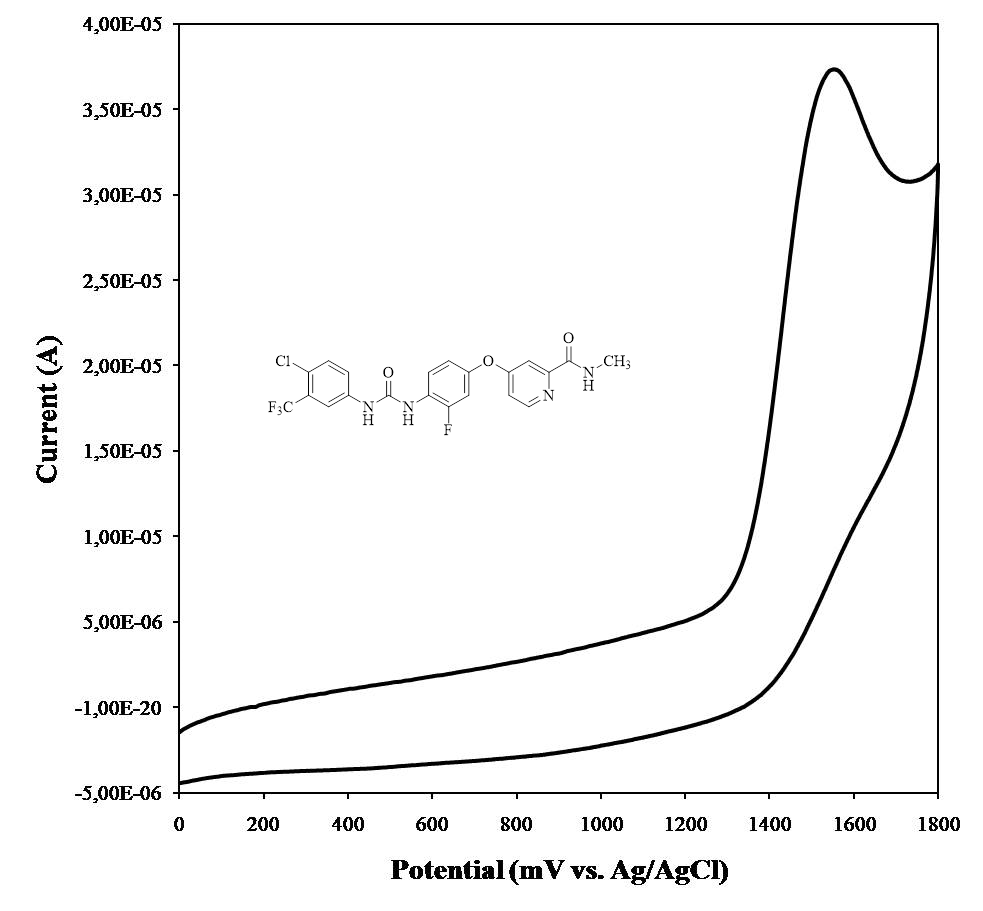
A novel tyrosine kinase inhibitor, regorafenib was electrochemically studied using a GC disc electrode in non-aqueous media. A well-resolved, irreversible, diffusion-controlled oxidation peak was obtained at 1.55 V in acetonitrile solution containing 0.1 M TBAClO4. Experimental conditions such as scan rate, indicate that two electrons play a role in the electrochemical oxidation of regorafenib. The recommended method was successfully applied to the determination of regorafenib in drug capsules.
KEYWORDS- Regorafenib
- cyclic voltammetry
- electrochemical oxidation