Organic Communications
A scientific open access journal in the field of synthetic organic chemistry and polymersLATEST ARTICLES
DBUHI3-catalyzed efficient synthesis of 1,4-dihydropyridines
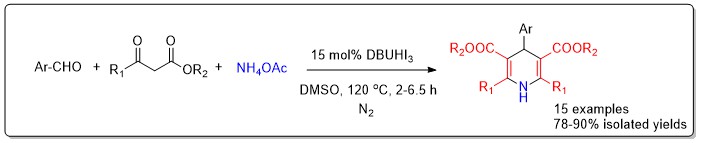
A process-optimized, one-pot multicomponent reaction catalyzed by DBUHI3 was developed, enabling the synthesis of diverse 1,4-dihydropyridines (1,4-DHPs), a class of bioactive nitrogen heterocycles from substituted benzaldehydes, ethyl acetoacetate, and ammonium acetate under mild conditions in DMSO. The methodology afforded good to excellent yields for a variety of aryl aldehyde substrates bearing both electron-donating and electron-withdrawing groups. Structural confirmation was achieved through IR, 1H NMR, 13C NMR, and HRMS analyses. This work not only summarizes key synthetic strategies but also provides a practical, sustainable route to the preparation of 1,4-DHPs, supporting further medicinal and synthetic exploration.
DOI http://doi.org/10.25135/org.2601.3780 Keywords 1,4-dihydropyridines (1,4-DHPs) DBUHI3 organocatalyst multicomponent reaction (MCR) DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.From the editor: meeting in the universal language of science in our 19th anniversary

Dear scientists and readers,
As the Organic Communications team, we proudly prepeare each new issue with the same excitement as with our very first issue for the last 19 years.
The first issue of 2026 represents both the change of the calendar and a new symbol of maturity and renewed vision of building a culture for the future.
Science Unites: A Shared Heritage
When I look back to 2008-2009 A significant moment stands out in my memory. At the beginning of our journey, among the very first manuscripts submitted to Organic Communications, one was from Palestine and another from Israel.
This coincidence demonstrates us the true mission of science: Science is universal; it has no country or homeland. Beyond the political borders, arguments and national concerns science is the most enduring bridge built for shared aspirations of humanity.
Scientists, as they have throughout history, continue to act together to carry this great heritage into the future.
We owe this to our past.
A Numerical Portrait of a 19-Year Journey
In line with this universal vision, our journal today reaches a global audience, featuring scientific contributions from 50 different countries spanning from Scandinavia to Australia. According to Web of Science data, the interest garnered by the 347 articles we have published to date is a clear indicator of our commitment to quality:
Global Impact: From a journey that began with just 4 citations, we have become a point of reference, receiving over 260 citations annually in 2024 and 2025.
Academic Trust: Web of Science data (as of 25.01.2026) shows that the vast majority of our 2,169 total citations (specifically 2,018) are not self-citations. A self-citation rate of only 6.7% proves how naturally and objectively our work is accepted worldwide. Articles published in Organic Communications have been cited in highly prestigious journals across various fields—primarily Chemistry, followed by Pharmacology, Pharmacy, Polymer Science, Biochemistry and Molecular Biology, Engineering, Infectious Diseases, Science and Technology, Material Science, Physics, and Environmental Sciences. This low self-citation rate is a refreshing data point in a publishing world often plagued by citation manipulation; it stands as a testament to the mutual trust between our authors, readers, and ourselves. We reaffirm our commitment to these principles and our role as a reliable pillar of the scientific community.
Respect for Scientific Effort: We believe that every effort is worth sharing. We have advocated that not only successful outcomes but also honestly reported "negative results" contribute to scientific methodology and prevent other researchers from wasting time and effort. Despite this inclusive approach, we have never compromised on academic rigor. Over the past 19 years, we have maintained a high scientific standard by providing constructive criticism and declining many more studies than we published. Recognizing the Peer Review system as a tool for collective learning and growth, our principled, transparent, and accountable decision-making process remains the greatest sign of our respect for all scientific contributors.
2026: A new model
As we are getting closer to our 19th year, we are excited to share significant developments with our readers:
i)New Layout: Our papers now will be more user friendly and professional; it will keep it easier for everyone to read in an efficient way.
ii) Continuous Publication Model: To keep pace with the accelerating nature of scientific publishing, we are adopting a continuous publication model. Accepted manuscripts will be rapidly converted into HTML and PDF formats, assigned a DOI, and published online without waiting for the completion of an issue. XML files will also be generated for indexing databases.
Acknowledgements
Since the beginning of this road, we have supported Open Access philosophy and with this same approach we will keep distributing the knowledge without institutional barriers.
I would like to extend my sincere gratitude to the members of our Editorial Board, to the more than one thousand authors from 50 countries who have contributed to our journal, and to our dedicated reviewers whose rigorous peer-review efforts have ensured the quality of our publications.
I wish you all a year driven by science, bringing peace and happiness to all humanity.
DOI http://doi.org/10.25135/org.2602.3801 Keywords Editorial DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.
Theoretical investigation of interactions between HIV-1 Tat and p53 proteins
HIV-1 Tat (transactivator of transcription) protein is the main arsenal of HIV, playing numerous roles during viral infection. This protein is intrinsically disordered, lacking well-defined secondary structures. Such structural plasticity allows HIV-1 Tat to interact with a wide range of proteins and biological molecules, ultimately leading to immune system collapse or severe tissue damage. Proteomic studies have previously revealed that p53, often referred to as the “guardian of the genome,” interacts with Tat through its tetramerization domain. Since p53 plays a pivotal role in determining cell fate, its interaction with Tat is of broad interest in the pathogenesis of HIV infection. Therefore, we investigated the complex formation between Tat and the tetramerization domain of p53 using molecular docking and molecular dynamics simulations. We believe that the results presented in this manuscript provide valuable insights for the development of novel therapeutic agents targeting the p53/Tat interaction.
DOI http://doi.org/10.25135/acg.oc.200.25.08.3618 Keywords HIV-1/2 Tat protein p53 molecular docking MD simulations protein-protein interaction DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.Synthesis and molecular docking studies of dispiropyrrolidine oxindole derivatives as a therapeutics agent against methicillin-resistant Staphylococcus aureus (MRSA)
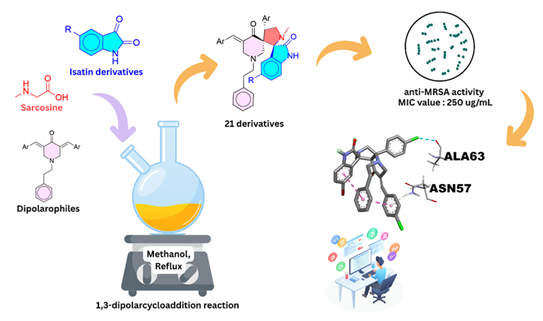
A series of new dispiropyrrolidine oxindole derivatives (8-10) were successfully synthesised with yield of 57 to 95% via one-pot 1,3-dipolar cycloaddition reaction of azomethine ylides and characterised by various spectroscopic techniques such as NMR, FT-IR, and HRMS. The compounds were evaluated for their activity against methicillin-resistance Staphylococcus aureus (MRSA). Among the compounds, compound 9f and 9g exhibit moderate activity against MRSA with minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of 250 µg/mL and 325 µg/mL, respectively. The binding energies and interactions of both compounds with S. aureus adhesion proteins such as sdrE, CIfA and FnBPA were further studied through molecular docking studies. Compound 9f (-8.5 ± 0.00 kcal/mol) showed a strong binding affinity than compound 9g (-7.5 ± 0.20 kcal/mol) particularly towards FnBPA adhesion protein. The molecular docking results revealed that the interactions between the compounds 9f and 9g with target proteins correlate with the observed MRSA inhibitory activity, highlighting their potential as promising lead candidates for anti-MRSA drug development.
DOI http://doi.org/10.25135/acg.oc.204.2511.3729 Keywords Dispiropyrrolidine oxindole anti-bacterial activity methicillin-resistant Staphylococcus aureus molecular docking in silico study DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.

