JOURNAL 1035
Bioorganic and Medicinal Chemistry Reports
Year: 2018 Issue: 1 January-December
p.6 - 15
Viewed 3811 times.
GRAPHICAL ABSTRACT
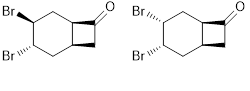
ABSTRACT
The electrophilic addition of bromine to bicyclo[4.2.0]oct-3-en-7-one in CHCl3 at 0 °C led to 60% yield of trans- and 30% yield of cis-dibromide. The structure of the synthesized molecules was determined using 1H and 13C NMR spectra. The biological activity of cis- and trans-dibromide was investigated in terms of antibiotic and toxic effects at cellular level using microbiologic and cytogenetic test, respectively. The antimicrobial activity of trans-dibromide9 and cis-dibromide10 was tested against Bacillus spizizenii ATCC 6633, Salmonella typhimurium ATCC 14028,Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 9027. Peripheral blood lymphocytes culture assay was used for determining the cytotoxicity of trans-dibromide 9 and cis-dibromide 10 substrates. Cis- and trans-dibromide showed antibiotic activity and the toxic effects of cis- and trans-dibromide were measured at cellular level by mitotic index as the cell kinetic parameter in a peripheral lymphocyte culture assay.
KEYWORDS- Dibromoketone
- biological activity
- antibiotic effect
- toxic effect
- mitotic index