JOURNAL 3179
Bioorganic and Medicinal Chemistry Reports
Year: 2024 Issue: 1 January-June
p.1 - 9
Viewed 2117 times.
GRAPHICAL ABSTRACT
ABSTRACT
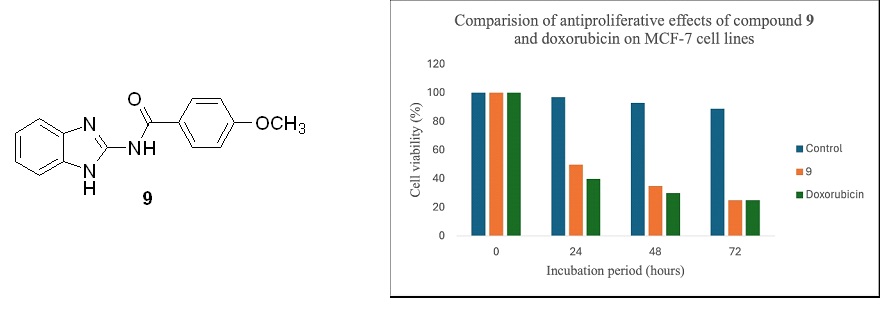
The strategic design of benzimidazole structures, akin to nucleotides, facilitates intricate interactions with amino acids within protein active sites. This structural scaffold, endowed with favorable pharmacokinetic profiles and lipophilic attributes, serves as a cornerstone in crafting potent pharmaceutical entities. In this study, a series of N-(1H-benzo[d]imidazol-2-yl)-substituted benzamides was meticulously synthesized and characterized through comprehensive spectroscopic analyses including IR, NMR, and elemental analysis. Leveraging the established pharmacophoric role of substituted benzimidazoles, renowned for their documented anti-proliferative properties, this study embarked on the synthesis of benzamides employing methoxy and substituted phenyl rings as key pharmacophores, known for their anticancer efficacy. Subsequent cytotoxicity evaluations using the MTT assay against MCF7 and normal mouse fibroblasts (L929) revealed compound 9 as the leading candidate, inducing significant cytotoxicity. This suggests its potential as a potent anticancer agent through apoptotic pathways. These findings highlight compound 9 as a promising molecular scaffold that requires careful optimization for the development of effective anticancer therapies.
KEYWORDS- Benzimidazole
- benzamide
- synthesis
- antiproliferative activity
- MTT assay
- structure-activity relationship