JOURNAL 3365
Bioorganic and Medicinal Chemistry Reports
Year: 2024 Issue: 2 July-December
p.33 - 39
Viewed 1566 times.
GRAPHICAL ABSTRACT
ABSTRACT
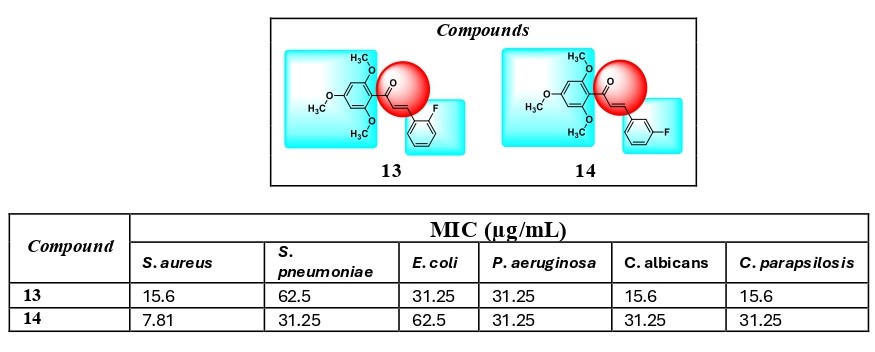
The rising resistance to antimicrobial drugs has highlighted the urgent need for discovering novel compounds with diverse mechanisms of action that can target both sensitive and resistant strains. To address this, we developed some chalcone analogs. In this study, a series of compounds (12-15) featuring fluoro and trifluoromethyl groups on the B ring were synthesized and evaluated for their antimicrobial properties. The positions of the substituents on the B ring were altered to assess their impact on antimicrobial activity. Compounds 13 and 14 demonstrated potent antibacterial activity (MIC = 15.6 and 7.81 μg/mL, respectively) against Staphylococcus aureus. Overall, our findings highlight Compounds 13 and 14 as promising scaffolds warranting further optimization for the development of effective antibacterial agents.
KEYWORDS- Chalcone
- synthesis
- antimicrobial activity
- MIC
- SAR