JOURNAL 1780
Journal of Chemical Metrology
Year: 2020 Issue: 2 July-December
p.88 - 105
Viewed 3214 times.
-
Adel Shehata

-
Abdulrahman Alaskar

-
Mohammed Alrashed

-
Abdullah Alosaimi

-
Fahad Alkharraa

-
Abdulrahman Alzahrani

GRAPHICAL ABSTRACT
ABSTRACT
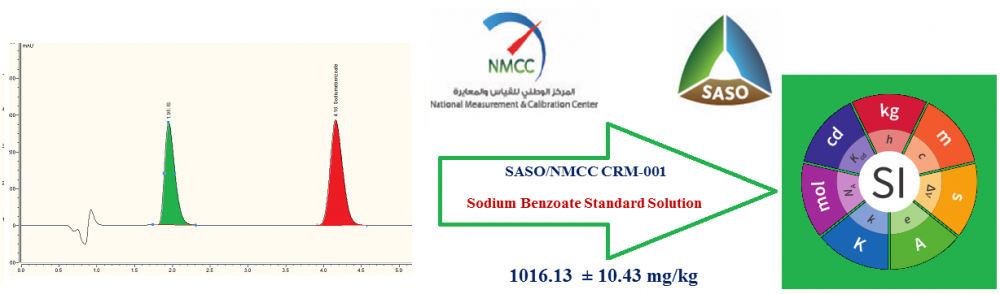
Sodium benzoate is one of the most widely used food perseverates and has to be used in regulated amounts to avoid its harmful health effects. Reviewing the scientific literature for traceability of the analytical measurement results of sodium benzoate in various food and drug applications to the SI units, it has been found that no scientific details of certification of sodium benzoate CRMs used for the calibration of measuring equipment are published. For this reason, the national metrology institute of Saudi Arabia (SASO/NMCC) certifies a sodium benzoate solution reference material. In this work, sodium benzoate was synthesized, purified and a batch solution reference material was prepared as 1014.47 mg/kg then homogenized and bottled. Homogeneity and stability of the candidate RM were assessed and the results obtained showed that the material is sufficiently homogeneous and stable. Characterization of the reference material was carried out by HPLC-UV, LC-MS/MS and UV-VIS-NIR spectrophotometer as three independent methods. The certified value was derived by combining data from the three methods using the weighted mean approach and was found 1016.13 mg/kg. The certified uncertainty was calculated as weighted uncertainty and was found 10.47 mg/kg (1.03%). Sources of this uncertainty were identified from the characterization, uchar, homogeneity, uhom, and long-term stability, ults, as well as the bias allowance, B.
KEYWORDS- CRM
- sodium benzoate
- homogeneity
- stability
- characterization
- certified value