JOURNAL 1744
Journal of Chemical Metrology
Year: 2020 Issue: 2 July-December
p.142 - 152
Viewed 3391 times.
GRAPHICAL ABSTRACT
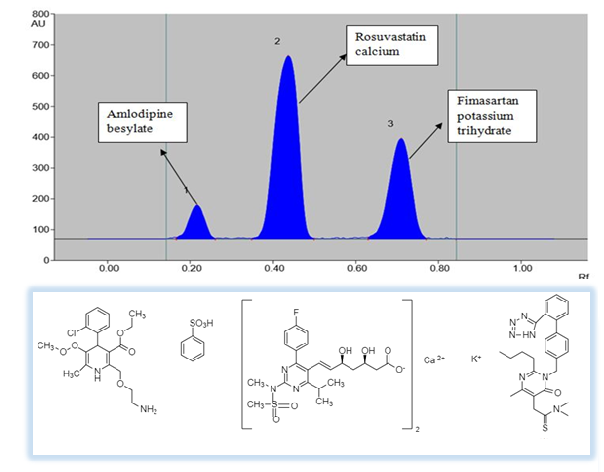
ABSTRACT
An accurate, sensitive, robust and precise high performance thin layer liquid chromatography method was developed based on ICH Q2 (R1) guidelines for estimation of novel combination of Amlodipine besylate, Rosuvastatin calcium and Fimasartan potassium in bulk and its synthetic mixture. Pre-coated silica gel aluminum plate 60 F254 was selected as the stationary phase and n-hexane, n-butanol, methanol, and Glacial Acetic Acid (5.7:2:2.3:0.1, v/v/v/v) was selected as mobile phase. All three drugs showing appreciable absorbance at the common wavelength of 242 nm were selected for quantification of Amlodipine besylate, Rosuvastatin calcium, and Fimasartan potassium, respectively. The method was validated for linearity, precision, accuracy, and robustness, limit of detection and limit of quantitation as per ICH parameters. The regression coefficients (r2) were found to be 0.9986, 0.9975 and 0.9988 for Amlodipine besylate, Rosuvastatin calcium, and Fimasartan potassium, respectively. The average percentage recovery of Amlodipine besylate, Rosuvastatin calcium and Fimasartan potassium were found to be 99.38-100-60%, 99.75-100.63%, 99.39-100%, respectively. Thin Layer Chromatographic method has prospective qualitative as well as quantitative applications for concurrent estimation of Amlodipine besylate, Rosuvastatin calcium and Fimasartan potassium in bulk and pharmaceutical dosage form.
KEYWORDS- HPTLC;
- amlodipine besylate (AML);
- rosuvastatin calcium(ROS);
- fimasartan potassium (FIM);
- validation