JOURNAL 2661
Journal of Chemical Metrology
Year: 2023 Issue: 1 January-June
p.42 - 53
Viewed 2935 times.
GRAPHICAL ABSTRACT
ABSTRACT
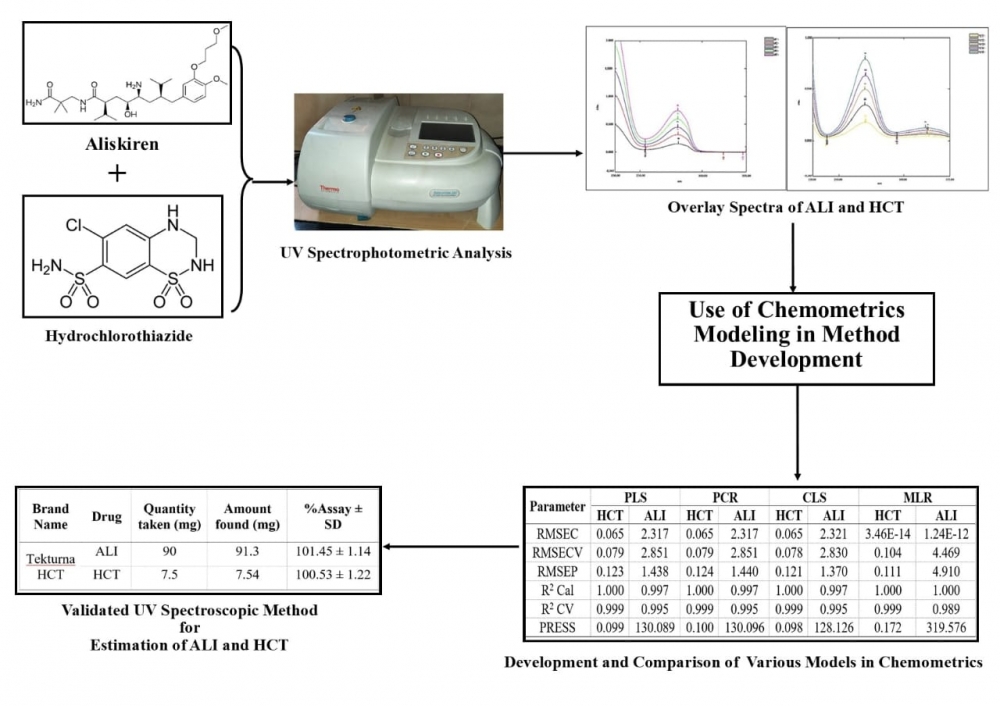
Aim of present research work was to develop and validate chemometrics assisted UV spectrophotometric method for simultaneous estimation of aliskiren and hydrochlorothiazide in bulk and their pharmaceutical dosage form. A five level two factor (2 Drugs) design was applied to get the 25 combinations of mixtures to be prepared. 16 set of mixtures were used for calibration and 9 set of mixtures were used for validation in wavelength range of 231-334 nm (wavelength interval λ=1 nm) in methanol. Various models like Classical Least Square (CLS), Multiple Linear Regression (MLR), Principal Component Regression (PCR) and Partial Least Square (PLS) were applied to the absorbance data obtained and among which CLS model was found to be best suited. Beer’s law was obeyed in concentration range 30 to 150 µg/mL for aliskiren and 2.5 to 12.5 µg/mL for hydrochlorothiazide. The method was then validated according to International Conference on Harmonization ICH Q2 (R1) and was found to be advantageous in terms of novelty and simplicity.
KEYWORDS- Chemometrics
- Aliskiren
- Hydrochlorothiazide
- Classical Least Square
- Inverse least squares
- Principal Component Regression