JOURNAL 2839
Journal of Chemical Metrology
Year: 2023 Issue: 2 July-December
p.215 - 224
Viewed 2392 times.
GRAPHICAL ABSTRACT
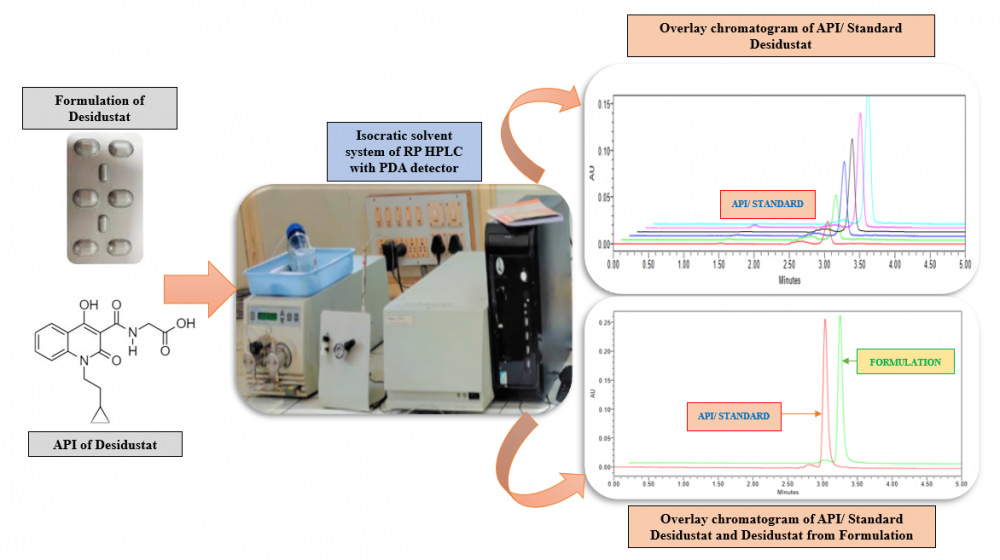
ABSTRACT
The hypoxia inducible factor prolyl hydroxylase inhibitor Desidustat is used to treat anemia linked to chronic kidney disease (CKD). For the estimation of Desidustat in bulk and commercial tablet formulation known as Oxemia, a precise, accurate, and sensitive reverse phase HPLC method has been developed and validated. The method described here was optimised with a Hypersil C18 (250 × 4.6 mm, 5 µm) column serving as the stationary phase and a mobile phase that included methanol: acetonitrile (80:20 v/v) added to the column at a flow rate of 1 mL/min. Using a photo diode array detector, Desidustat was detected at an analytical wavelength of 230 nm. With a correlation coefficient of 0.9989, the developed method was found to be linear in the concentration range of 1–6 µg/mL. Every parameter listed in the in International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Quality 2 (Revision 1) guideline was verified for the described method.
KEYWORDS- Desidustat
- HPLC
- method validation