JOURNAL 2949
Journal of Chemical Metrology
Available Online: January 11,2024
p.1 - 9
http://doi.org/10.25135/jcm.2311.2949 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 670 times.
GRAPHICAL ABSTRACT
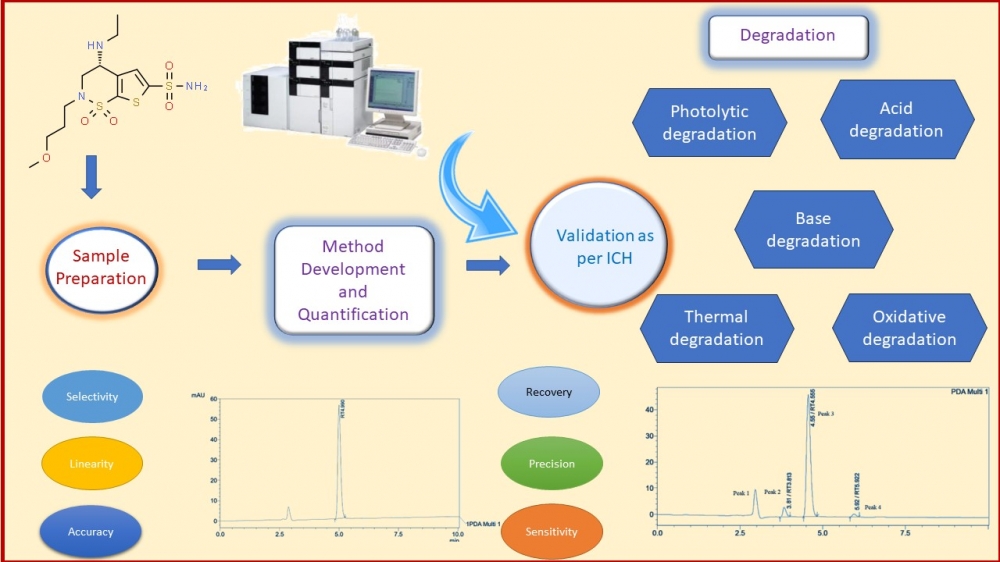
ABSTRACT
This study outlines the development, optimization, and validation of a robust reverse-phase high-performance liquid chromatography (RP-HPLC) technique for the precise quantification of Brinzolamide in ophthalmic products, aligning International Council for Harmonization (ICH) guidelines. The method employed a Phenomenex (C18) (250×4.6mm) column with 5μm particle size as the stationary phase, a 1 mL/min flow rate for the mobile phase (composed of acetonitrile: water 35:65 v/v, pH adjusted to 3 with orthophosphoric acid), and detection at 254 nm. Under these conditions, Brinzolamide displayed Rt of 4.9 minutes. The validation process, following ICH standards, exhibited excellent linearity within the 5–30 μg/mL concentration range, with a limit of detection at 0.22 μg/mL and a limit of quantification at 0.67 μg/mL. Recovery rates from ophthalmic formulations fell between 98.3%-101.08%, indicating high accuracy. Accelerated stability assessments conducted over three months revealed content retention between 98.2%-100.9%, affirming the product's stability. Additionally, Brinzolamide withstood various stress conditions without interference in quantification, as the degradation products had distinct retention times from the pure drug, offering excellent resolution. In conclusion, this RP-HPLC method is suited for routine quality control analysis of Brinzolamide in commercial ophthalmic preparations, due to its specificity, accuracy, precision, and sensitivity, aligning perfectly with ICH guidelines.
KEYWORDS- Brinzolamide
- RP-HPLC method
- accelerated stability study
- force degradation
- degradation products