JOURNAL 1844
Organic Communications
VOLUME & ISSUE
Year: 2021 Issue: 1 January-March
Year: 2021 Issue: 1 January-March
PAGES
p.92 - 96
p.92 - 96
STATISTICS
Viewed 2811 times.
Viewed 2811 times.
GRAPHICAL ABSTRACT
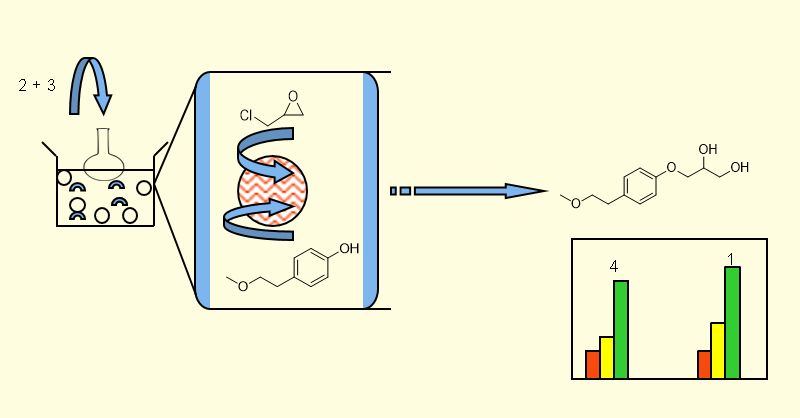
ABSTRACT
Safety of the drug molecule is dependent on the various impurities formed during chemical transformation which may cause drug product instability, decreased product performance, loss in potency, and/or formation of potentially genotoxic impurities. Therefore, it is essential to identify and quantify the impurities generated during the drug development stage. Herein, we describe the synthesis and characterization of Impurity D of Metoprolol, 3-[4-(2-methoxyethyl)phenoxy]propane-1,2-diol (1), reported in European Pharmacopeia. The synthesis of impurity D has been accomplished in two steps; starting from Epichlorhydrin under three different set of conditions.
KEYWORDS- Keywords: Impurity D
- Metoprolol
- synthesis
- characterization
- ultrasonic waves