JOURNAL 2279
Organic Communications
Year: 2022 Issue: 2 April-June
p.167 - 183
Viewed 2933 times.
GRAPHICAL ABSTRACT
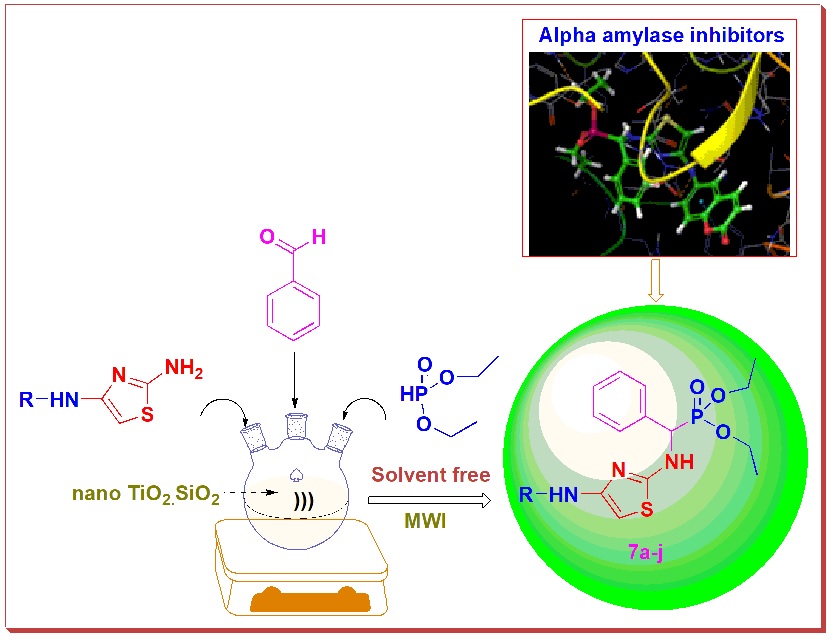
ABSTRACT
Using microwave irradiation, an effective and greener method for the synthesis of α-aminophosphonates via Kabachinic-Fields reaction in a solvent-free environment is devised. An in silico ADMET and molecular docking analysis was performed on all of the compounds to get insight into their drug likeliness behaviour as well as their capacity to block the enzyme α-amylase (PDB ID: 3IJ8). The compounds with the highest binding affinity and pharmacokinetic properties were developed. The newly created compounds were spectroscopically examined to establish their structure, and all of them were tested for in vitro α-amylase inhibitory action. The compounds 7j (IC50: 99.9±0.3 μg/mL) and 7e (IC50: 102.0±0.7 μg/mL) showed stronger inhibitory efficacy than acarbose, the reference medication. The compounds 7g (IC50, 106.7±0.4 μg/mL), 7d (IC50, 108.4±0.3 μg/mL), 7h (IC50, 115.0±0.4 μg/mL), and 7f (IC50, 119.2±0.4 μg/mL) have shown to inhibit the target enzyme significantly.When compared to the reference drug, Acarbose (IC50, 102.6±0.8 μg/mL), all of the remaining compounds showed modest to good inhibition with IC50 values ranging from 125.6±0.6 to 152.7±0.2 μg/mL. The findings revealed that the vast majority of these drugs have strong α-amylase inhibitory action.
KEYWORDS- α-aminophosphonates
- ADMET
- molecular docking
- α-amylase
- Acarbose