JOURNAL 3626
Organic Communications
Available Online: November 03,2025
p.1 - 18
http://doi.org/10.25135/acg.oc.202.2508.3626 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 7 times.
-
Selvakumar Sabapathi

-
Babai Mahdi

-
Mohamad Hafizi Abu Bakar

-
Andrey A. Mikhaylov

-
Mohammad Tasyriq Che Omar

-
Azeana Zahari

-
S. Yaallini Sukumaran

-
Mohamad Nurul Azmi

GRAPHICAL ABSTRACT
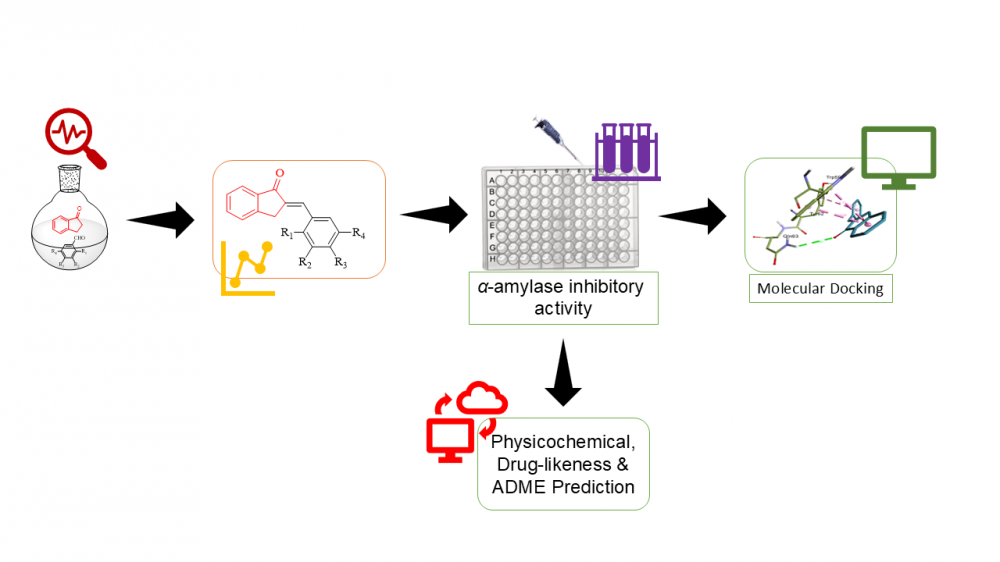
ABSTRACT
In search for novel antidiabetic agents, a new series of substituted 2-benzylidene-1-indanone derivatives were synthesized via crossed Adol condensation reaction. The structures of the synthesized compounds were determined using various spectroscopic techniques, including HREIMS, FTIR, and NMR. The enzyme inhibitory activities of the target analogues were assessed using in vitro assays. The tested compounds demonstrated inhibitory potential against α-amylase, as indicated by their IC50 values ranging from 17.7 to 28.2 µM as compared to standard drug acarbose with IC50 value of 30.2 ± 1.9 µM. Furthermore, molecular docking study was conducted to elucidate the binding interactions of the compounds within the α-amylase enzyme binding pocket (PDB ID 2QV4). The results of molecular docking studies indicated that compounds 3m, 3c, 3d has the lowest binding energy (-9.8, -9.3 and -9.4, respectively). The structure-activity relationship (SAR) analysis revealed that alteration in the inhibitory activities of α-amylase enzymes was provided by distinct types of substituents attached to either ortho- or para positions of the phenyl group. The combined SAR and docking results highlight the importance of para-position substitution on ring A for optimal activity, particularly when introducing moderately electron-withdrawing groups such as chlorine, fluorine, and bromine. Thus, in the pursuit of developing newer antidiabetic agents, the in silico ADME prediction was carried out with promising physicochemical, drug likeness and ADME properties which indicated that some compounds were considered drug-like as they do not violate any of the rule-based filters of Lipinski.
KEYWORDS- 2-benzylidene-1-indanone derivatives
- Aldol condensation reaction
- α-amylase inhibitor
- Molecular docking
- ADME properties