JOURNAL 3531
Organic Communications
Available Online: August 30,2025
p.1 - 44
http://doi.org/10.25135/acg.oc.194.2505.3531 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 208 times.
GRAPHICAL ABSTRACT
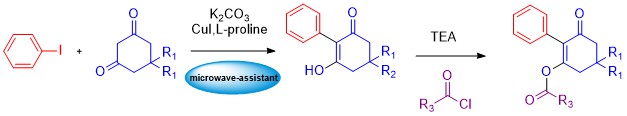
ABSTRACT
This review focuses on the design, synthesis, and biological evaluation of novel heterocyclic compounds derived from β-diketones and cyanomethylene reagents through multicomponent and green synthetic methodologies. The study encompasses a wide range of heterocyclic scaffolds, including xanthene, chromene, chromenone, coumarin, acridine, quinoline, thiazole, thiophene, and spiro-heterocycles containing nitrogen, oxygen, and sulfur. A variety of catalysts were employed such as DBSA, P-TSA, NbCl₅, and nano-magnetic composites like CuFe₂O₄/chitosan to optimize reaction conditions for eco-friendly and high-yielding transformations. The developed synthetic strategies included one-pot, microwave-assisted, and solvent-free techniques, resulting in efficient routes to complex molecular architectures. The biological activity of the synthesized compounds was extensively screened, with several candidates exhibiting promising antimicrobial, antifungal, anticancer, and kinase-inhibitory properties. Structure-activity relationship (SAR) studies indicated that specific heteroatom substitutions enhanced biological potency, particularly in xanthene and chromenoquinoline derivatives. This work contributes to advancing heterocyclic chemistry by introducing new reaction pathways, novel molecular frameworks, and bioactive agents with potential pharmaceutical applications.
KEYWORDS- Camphor
- pyrazole
- camphor dimethyl DL tartrate;
- thiazole
- biological activity