JOURNAL 3058
Records of Agricultural and Food Chemistry
Year: 2024 Issue: 3 Special Issue: Abstracts 3rd. TCS, International Food Chemistry Congress February 29-March 03,2024 Antalya Türkiye
p.24 - 24
Viewed 209 times.
GRAPHICAL ABSTRACT
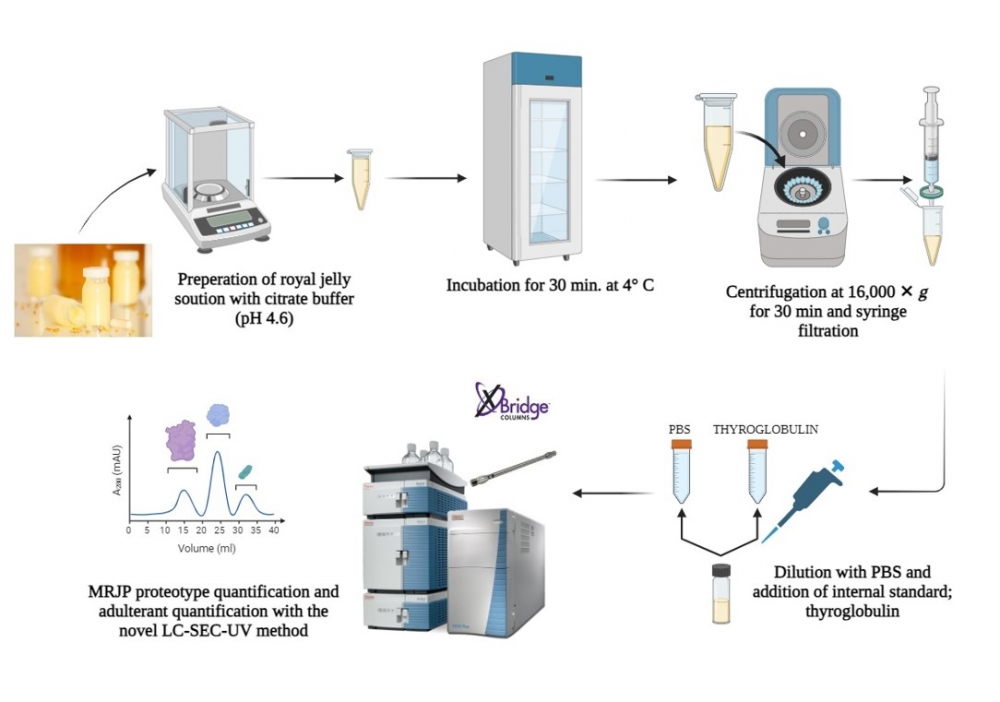
ABSTRACT
Polymorphism-derived proteotypes named MRJP-1 oligomer (Apisin), MRJP-2, MRJP-3, and MRJP-1 monomer (Royalactin) are predominant in RJ and the bioefficacy of the products greatly vary depending on their profile [1]. Legislations suggest following the total protein concentration and 10-HDA contents of the RJ products as the bioactive constituents. However, each MRJP variant has distinguished health benefits and MRJPs undergo a rapid structural change depending on storage conditions and shelf life, and their effectiveness may be modified or reduced [2]. Therefore, measuring the individual concentrations of each intact MRJP instead of the total protein and inspecting the modifications is crucial. In this study, it was aimed to develop a practical analytical method for the absolute quantification of MRJP variants. A novel MRJP purification workflow was also presented to obtain authentic standards for Native-LC-SEC-UV method development and for constructing the calibration plots. The supernatant of RJ proteome extract was subjected to tandem purification at FPLC performing cation, anion exchange, and size exclusion (SEC) respectively. Subsequently, obtained fractions were desalted and concentrated and purities were checked by SDS-PAGE. Protein annotation of each purified MRJP was accomplished by bottom-up proteomic assay. In-gel digested purified proteins were transferred to UHPLC fractionation and Orbitrap HRMS operated in FS-ddMS2 acquisition. Data was processed at Proteome Discoverer and sequences were retrieved from UniProtKB. Real sample analysis (n=15) was performed using the developed native mode LC-SEC-UV method. Thyroglobulin was spiked for normalization and the RJ proteome was extracted using a citrate buffer (pH 5.6). The LC system was operated under isocratic flow and all MRJP critical pairs were chromatographically resolved via SEC column. Ovalbumin, milk powder, and melamine as potential adulterants were simultaneously screened, thus mimicked RJ samples could also be reported. HRMS data in agreement with SDS-PAGE suggested that Apisin is mainly characterized at 250 kDa, meanwhile, MRJP-3, MRJP-2, and Royalactin are identified roughly at 70, 53, and 57 kDa respectively. LC-SEC presented an accurate quantification for all MRJP proteoforms. MRJP-2 and MRJP-3 results were between 1.1-29.5 mg, and 0.6-22.8 mg respectively. Royalactin was quantified between 0.4-10.4 mg while Apisin was found between 1.2-78.2 mg. It was determined that storage conditions dramatically affect the protein composition and Apisin was the most fragile variant. Higher temperatures, inefficient lyophilization, or improper packaging promote Maillard reactions resulting in higher glycation-derived protein loss. Furthermore, it was observed that the lyophilization process can induce MRJP3 to be fragmented and Royalactin to be oligomerized. None of the samples contained any adulterants, but as the bee products rescript will come into effect soon, it is considered to be vital to analyze the potential adulteration. These results proved that adapting the novel LC-SEC-UV method into the QC workflows can reliably indicate the quality of the final RJ products.
KEYWORDS- Royal Jelly
- protein
- size exclusion
- quality
- HPLC