JOURNAL 1053
Records of Natural Products
Year: 2019 Issue: 4 July-August
p.287 - 295
Viewed 4322 times.
-
Arif Ullah, Ghias Uddin, Mamoon Ur Rashid, Ismail Ismail, Nasruddin, Bina Shaheen Siddiqui, Zahoor Ullah, Muhammad Alamzeb
GRAPHICAL ABSTRACT
ABSTRACT
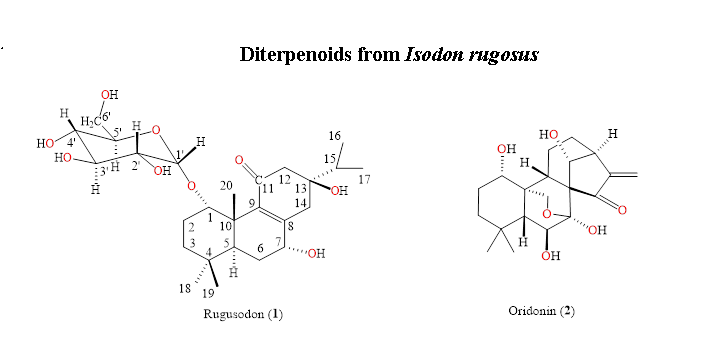
The methanolic extract of the roots of Isodon rugosus were subjected to chromatographic separation, yielded two diterpenes: a new compound named rugosodon 1 and a known compound oridonin 2. The new compound 1 was elucidated as 1-O-α-D-glucopyranosyl-7α,13β-dihydroxyabieta-8(9)-en-11-one and the known compound 2 as 7α,20-epoxy-1α,6β,7,14-tetrahydroxy-kaur-16-en-15-one (oridonin), based on the chemical hydrolysis, physicochemical and spectroscopic data of IR, ESIMS, EIMS, 1D, and 2D NMR. The compounds 1-2 were subjected to bioassay studies of cytotoxicity and α-glucosidase inhibition potential. Rugosodon 1 showed significant potential of α-glucosidase inhibition with IC50 0.453 mg/mL, as compared to the standard acarbose (IC50 0.921 mg/mL). The compounds 1-2 failed to show any significant results for the cytotoxic screening against the three human cancer cell lines (NCI-H460, HeLa and MCF-7).
KEYWORDS- Abietane
- diterpene
- glycoside
- Isodon rugosus
- rugosodon
- oridonin