JOURNAL 1940
Records of Natural Products
Year: 2021 Issue: 5 September-October
p.368 - 379
Viewed 2758 times.
GRAPHICAL ABSTRACT
ABSTRACT
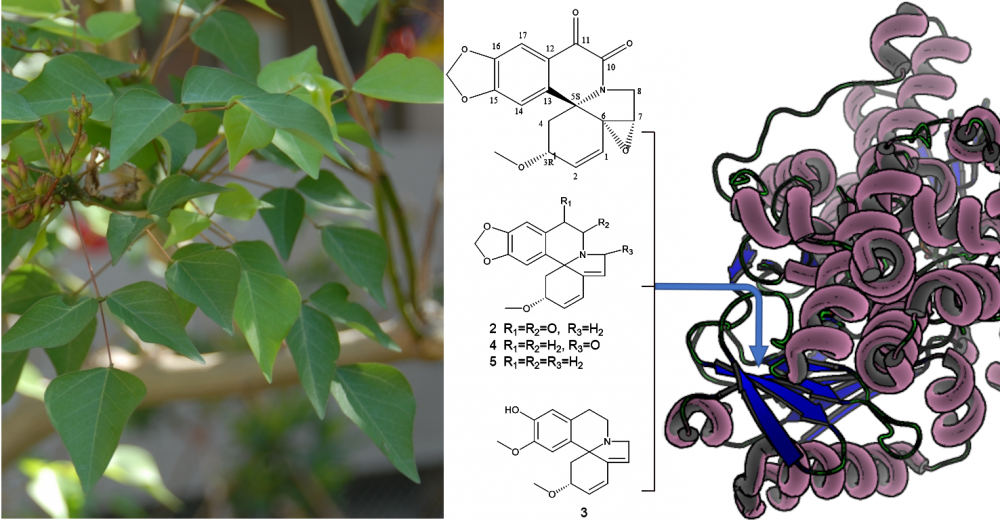
One new, 10, 11-dioxo-6,7α-erythraline epoxide (A1) and four known erythrinan alkaloids 10, 11-dioxo-erythraline (A2), erysodine (A3), 8-oxo-erythraline (A4) and erythraline (A5) were isolated from the 70% methanolic extract of stems of E. Corallodendron (Fabaceae). The isolated compounds were elucidated by exploiting 1D/2D NMR, and HR-ESI-MS analysis. The absolute configuration of A1 was determined by electronic circular dichroism (ECD). Mono Amine Oxidase inhibitory activity of the isolated alkaloids was investigated in vitro using kynuramine deamination assay on recombinant human MAO-A and B enzymes. The binding modes were predicted by molecular docking and the structure activity relationships of erythrinans were then evaluated. All isolated alkaloids demonstrated preferential activity against MAO-B. A1 displayed the highest potency and selectivity against MAO-B with IC50 of 25.18 μM, (SI >3.97). The selective inhibition exhibited by erythrinan alkaloids against MAO-B is in line with the expected biological impact of Erythrina in the treatment of neurodegenerative diseases and presents this chemical class as promising leads for managing AD and PD diseases.
KEYWORDS- Erythrina corallodendron
- alkaloids
- Alzheimer’s disease
- Parkinson’s disease
- selective MAO-B inhibitors
- docking