JOURNAL 2330
Records of Natural Products
Year: 2023 Issue: 1 January-February
p.99 - 105
Viewed 3892 times.
-
Na Sun

-
Tingting Du

-
Yifang Zhang

-
Yiming Luo

-
Xinyu Ma

-
Ru Zhang

-
Xinru Wu

-
Huanhuan Yang

-
Miao Zhou

-
Penghua Shu

-
Jihong Huang

GRAPHICAL ABSTRACT
ABSTRACT
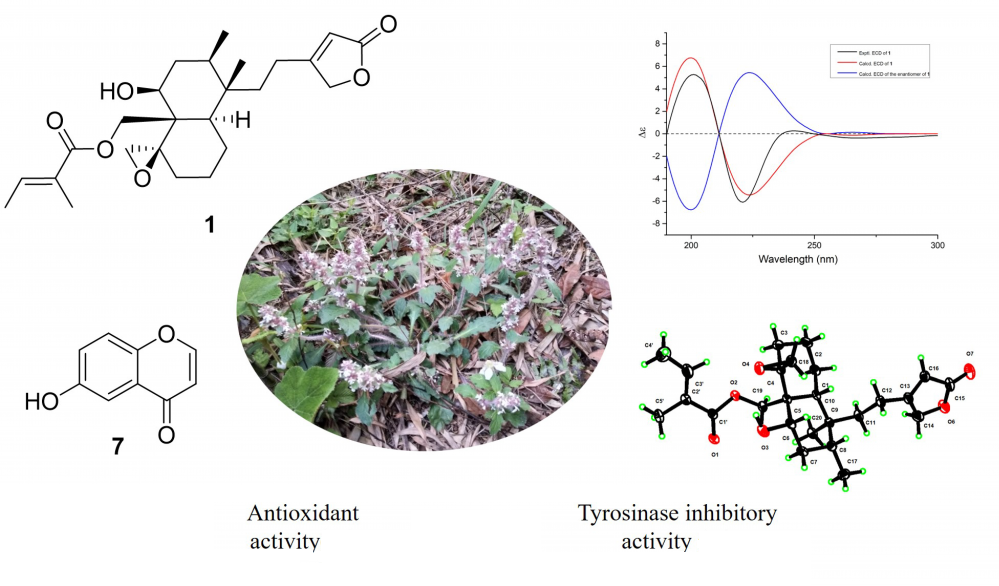
One neo-clerodane diterpenoid (1), two abietane diterpenoids (2 and 3), three flavonoids (3–6), one C6-substituted chromone derivative (7), one phenylpropanoid (8), as well as one chain fatty acid (9) were isolated from the whole plants of Ajuga nipponensis. Their structures were established by detailed analysis of the HRESIMS, 1D and 2D NMR, UV, and IR. The absolute configuration of compound 1 was firstly determined by ECD calculation and single crystal diffraction. The NMR data of compound 9 are reported for the first time. Compounds 4, 7–9 were isolated from this medicinal plant for the first time, and 4, 9 were first discovered from natural products. The isolates 1–7 were tested for their antioxidant and mushroom tyrosinase inhibitory activities. Most of them showed moderate antioxidant activities. Compounds 1, 4, 5, and 7 exhibited obvious antioxidant activities at 50 μM, with % inhibition values of 32.37±0.75%, 25.30±0.66%, 28.76±0.64%, 31.06±0.46%, respectively, with L-ascorbic acid used as the positive control (71.29±0.34%). Compound 7 exhibited significant inhibitory activities against mushroom tyrosinase (%inhibition values of 16.94±1.28%), with arbutin used as the positive control (29.9±0.99%).
KEYWORDS
- Ajuga L
- Ajuga nipponensis
- diterpenoid
- flavonoid
- antioxidation
- tyrosinase inhibitory activity