JOURNAL 2573
Records of Natural Products
VOLUME & ISSUE
Year: 2023 Issue: 2-March-April
Year: 2023 Issue: 2-March-April
PAGES
p.335 - 342
p.335 - 342
STATISTICS
Viewed 3349 times.
Viewed 3349 times.
GRAPHICAL ABSTRACT
ABSTRACT
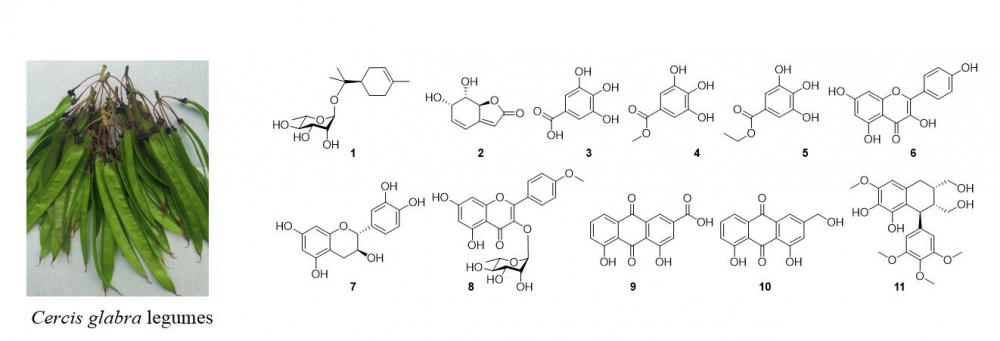
A new monoterpene rhamnoside, (+)-(R)-α-terpineol α-L-rhamnoside (1), along with ten known compounds (2–11) were obtained after purification of the ethanol extract of Cercis glabra legumes. Their structures were elucidated by spectroscopic evidence including NMR, optical rotatory dispersion (ORD), HR-ESI-MS and chemical hydrolysis. In the acetylcholinesterase inhibitory assay, compounds 3 and 8 showed high percentages of inhibition which were comparable to the activity of donepezil (a commercial drug, the positive control), and exhibited IC50 values of 0.488, 0.391 mg/mL. These bioactive components could be promising acetylcholinesterase inhibitors.
KEYWORDS- Cercis glabra
- monoterpene
- acetylcholinesterase
- acid hydrolysis