JOURNAL 2454
Records of Natural Products
VOLUME & ISSUE
Year: 2023 Issue: 3 May-June
Year: 2023 Issue: 3 May-June
PAGES
p.561 - 565
p.561 - 565
STATISTICS
Viewed 3422 times.
Viewed 3422 times.
GRAPHICAL ABSTRACT
ABSTRACT
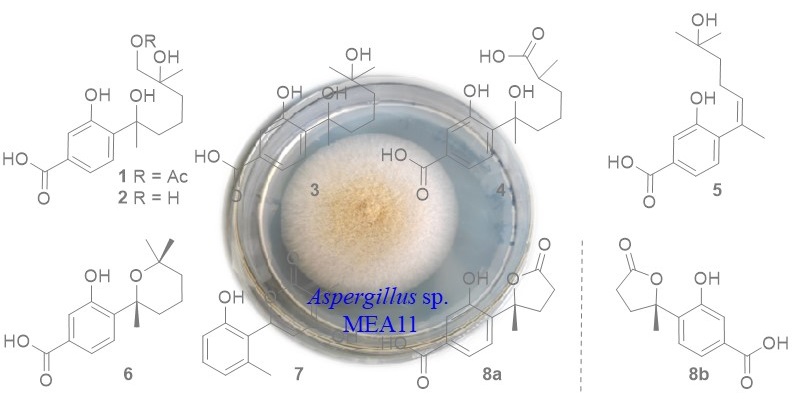
In our work, the deep sea sediment-derived fungus Aspergillus sp. MEA11 was examined for secondary metabolites. Chromatographic separations resulted in the identification of a new phenolic bisabolane (1) and seven known analogs (3-7 and 8a, 8b). The structures were determined by 1H, 13C NMR, and MS data. The known compounds were identified to be 11,12-dihydroxysydonic acid (2), hydroxysydonic acid (3), aspergoterpenin B (4), engyodontiumone J (5), sydowic acid (6), penicipyran A (7), 1-hydroxyboivinianic acid (8). The NMR data of 7 in methanol-d4 were reported for the first time. Compounds 6-8 exhibited inhibitory effect against α-glucosidase with IC50 values of 176, 89, 232 uM, which were more active than the positive control acabose.
KEYWORDS- Phenolic bisabolanes
- Aspergillus sp.