JOURNAL 3446
Records of Natural Products
Available Online: July 26,2025
p.1 - 8
http://doi.org/10.25135/rnp.533.2502.3446 (DOI number will be activated after the manuscript has been available in an issue.)
Viewed 147 times.
GRAPHICAL ABSTRACT
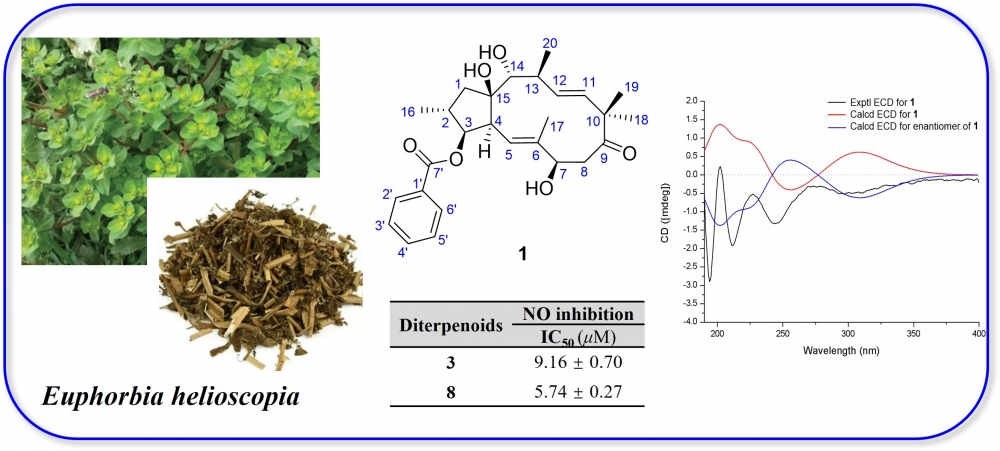
ABSTRACT
Phytochemical investigation of 70% ethanol extract of Euphorbia helioscopia led to eight diterpenoids, including four jatrophane-type (1–4), three abietane-type (5–7) and one lathyrane-type (8). Among them, (5E,11E)-3β-benzoyloxy-7β,14α,15β-trihydroxyjatropha-9-one (1) was a new jatrophane diterpenoid. An extensive array of spectral analyses, including HR-ESI-MS, IR, UV, 1D and 2D NMR, as well as quantum chemical calculations of ECD curve, were utilized for their structural elucidation. Bioassay results showed that compounds 2–3 and 6–8 could significantly inhibit NO release on LPS-induced RAW 264.7 cells, especially for jatrophane diterpenoid 3 and abietane diterpenoid 8 that showed stronger activities with the IC50 values of 9.16 ± 0.70 and 5.74 ± 0.27 μM.
KEYWORDS- Euphorbia helioscopia
- diterpenoids
- anti-inflammatory