JOURNAL 1465
Journal of Chemical Metrology
Year: 2019 Issue: 2 July-December
p.39 - 46
Viewed 3632 times.
GRAPHICAL ABSTRACT
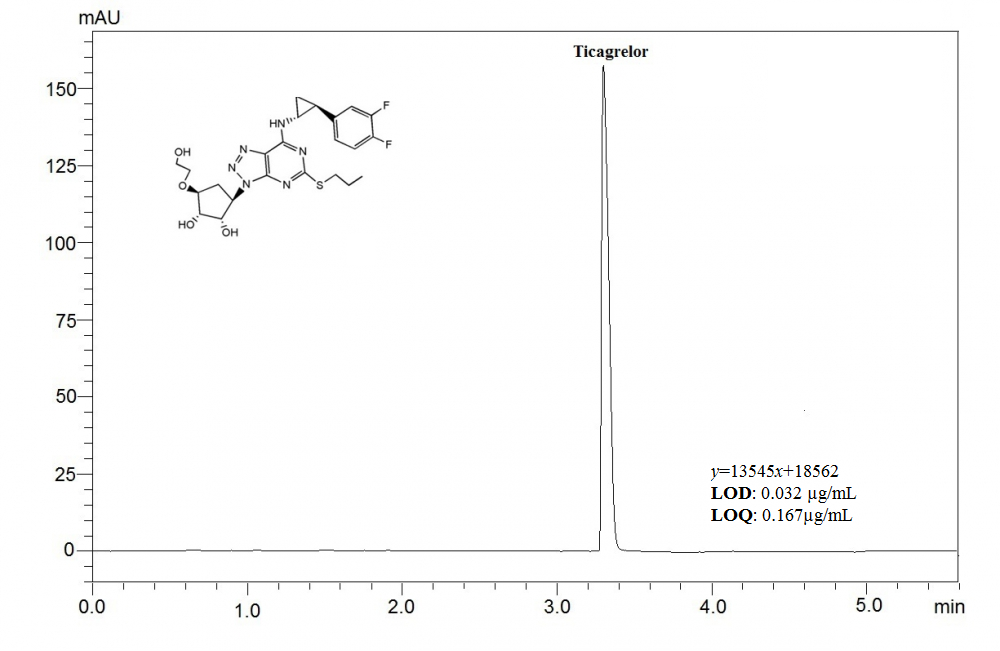
ABSTRACT
This article aims to present a sensitive, and precise stability-indicating ultra fast liquid chromatographic method, which was developed to determine ticagrelor (TCG) in pharmaceutical formulations and spiked plasma samples. Chromatographic separation was achieved under isocratic elution by using a C18 column (100 × 4mm, 3μm) and a mixture of acetonitrile and phosphoric acid solution (adjusted to pH 3.0 using triethylamine) (55:45, v/v) at a flow rate of 0.7 mL per minute. The analyte was detected at a wavelength of 254 nm, using a photodiode array detector (PDA). The retention time for TCG was found out to be about 3.5 min. As required by the International Conference on Harmonization guidelines, the drug was exposed to different stress conditions; including hydrolysis (acid, alkaline), oxidation, photolysis and thermal degradation. This currently developed method was validated by evaluating the specificity, linearity, precision, accuracy, robustness, and system suitability. The method was determined to be linear in a drug concentration range of 0.5–200 μg/mL with the correlation coefficient of 0.9996. The proposed method was applied successfully to the analysis of TCG in spiked human plasma with good recovery. The method can successfully applied for determination of TCG in pharmaceutical formulation and spiked plasma samples.
KEYWORDS- Ticagrelor
- UFLC
- stability-indicating
- degradation
- method validation
- plasma samples