JOURNAL 1994
Journal of Chemical Metrology
Year: 2021 Issue: 1 January-June
p.25 - 37
Viewed 3017 times.
GRAPHICAL ABSTRACT
ABSTRACT
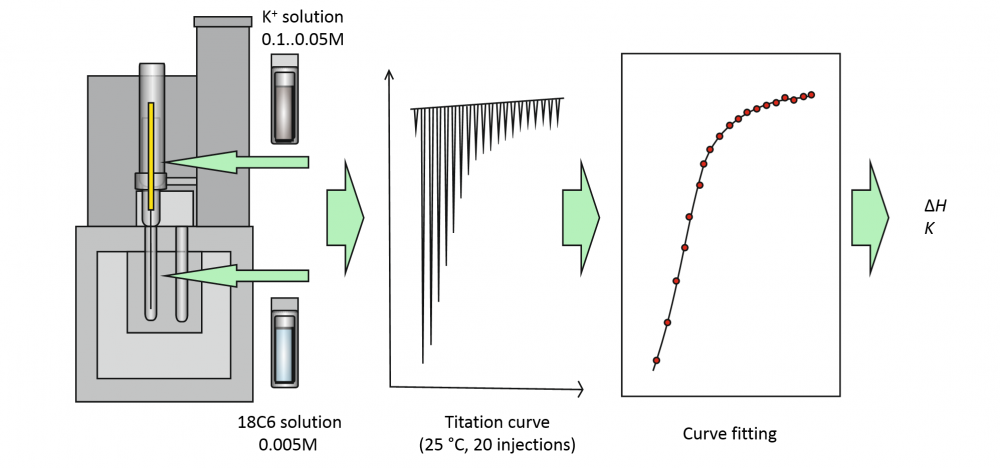
Combination of low concentration, low binding affinity and small solution volume is increasingly more common in calorimetric studies of host-guest interaction, but it leads to very small enthalpy and entropy effects, which are difficult to measure accurately. The aim of this study was to evaluate the reliability of enthalpy determination under such conditions using isothermal titration calorimetry (ITC) with a small-volume microcalorimeter and to evaluate the expected accuracy (expressed as measurement uncertainty) on the example of a simple 1:1 binding reaction of 18-crown-6-ether with K+ cation. The investigation focused on whether or not it is possible to get meaningful results from experiments on such a system with low binding constant, low concentration (logK ≈ 2), low Wiseman “c” parameter values (c < 1) and low total heat released in the studied system. A thorough estimation of measurement uncertainty was performed using the component-by-component (the “classical” GUM) approach to estimate the uncertainties of experimentally determined heat effects at individual titration points and applying those uncertainties to data fitting to obtain the K and ΔH values for the studied reaction. We found that it was possible to determine the reaction enthalpy ΔH of -26.6 kJ/mol with standard uncertainty of 1.1 kJ/mol. If we assume similar uncertainties for the other authors, the ΔH value found in this work is in good agreement with the majority of the literature ΔH values and those are in general in agreement with each other.
KEYWORDS- Isothermal titration calorimetry
- Wiseman c parameter
- measurement uncertainty
- studying the binding of low-affinity systems