JOURNAL 2977
Journal of Chemical Metrology
Year: 2023 Issue: 2 July-December
p.238 - 248
Viewed 1956 times.
GRAPHICAL ABSTRACT
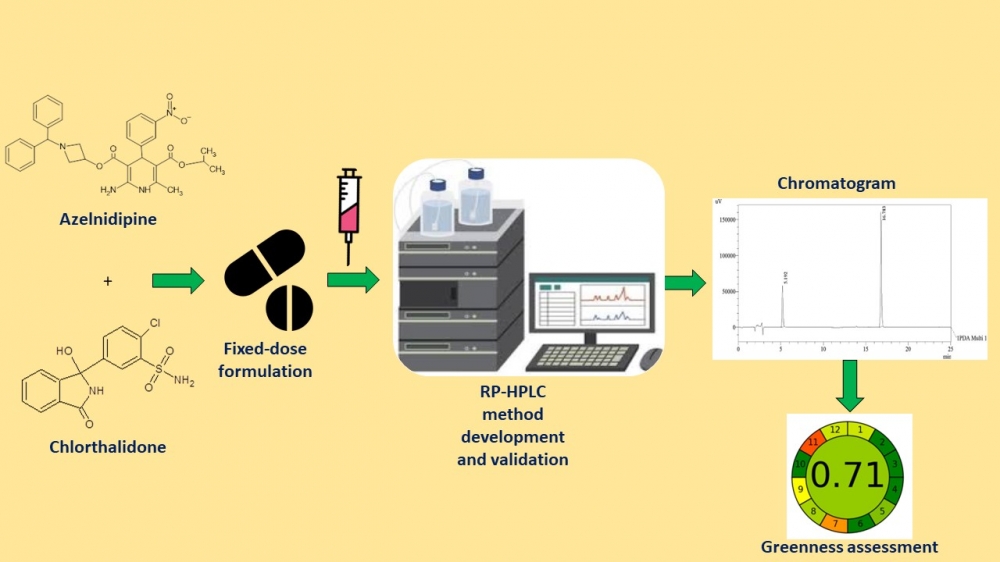
ABSTRACT
Fixed-dose drug combinations promote compliance and optimize prescription schedules. Recently, the FDA approved a film-coated tablet containing azelnidipine (8 mg) and chlorthalidone (6.25 or 12.5 mg) for the treatment of hypertension. A novel gradient mode high-performance liquid chromatography (HPLC) technique was developed and rigorously validated to enable the routine analysis of a fixed-dose combination. Materials and Methods: The stationary phase employed in this study was a Phenomenex Luna C8 column (250 × 4.6 mm, 5 µm particle size). The mobile phase consisted of a mixture of acetonitrile and water, with the addition of 0.1 percent formic acid, employed in a gradient mode. A typical detection wavelength of 256 nm was utilized for both compounds. The validation of this method adhered to the performance parameters outlined in the ICH Q2 (R1) guidelines. Results: This method successfully analyzed azelnidipine (99.58% w/w) and chlorthalidone (100.25% w/w) in a synthetic mixture prepared from their respective commercial tablet formulations. This process was executed to ensure the absence of plagiarism in the revised text. Conclusion: Thus, the technique inferred is ideal for routine examination of fixed-dose tablet formulation.
KEYWORDS- Azelnidipine
- chlorthalidone
- hypertension
- liquid chromatography
- synthetic mixture