JOURNAL 2936
Journal of Chemical Metrology
Year: 2024 Issue: 1 January-June
p.41 - 49
Viewed 2537 times.
GRAPHICAL ABSTRACT
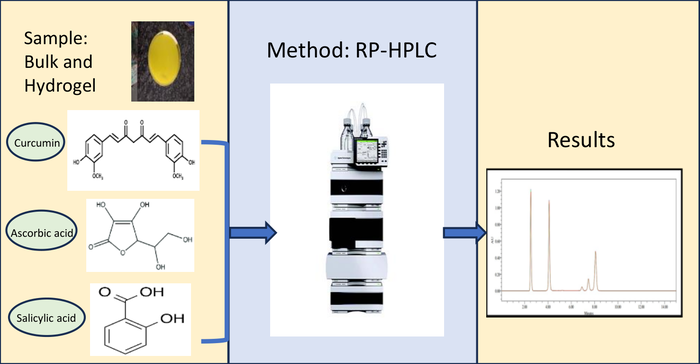
ABSTRACT
A precise and specific reverse phase high-performance liquid chromatographic method has been developed and validated to quantify curcumin, ascorbic acid, and salicylic acid in both bulk and hydrogel. Utilizing a Hypersil BDS C18 column and an isocratic mode, the mobile phase comprised of a mixture of 0.1% orthophosphoric acid and acetonitrile (50:50 v/v). The calibration range spanned concentrations of 100 - 300 µg/mL for curcumin, 50 - 150 µg/mL for ascorbic acid and 50 - 150 µg/mL for salicylic acid. The specificity of the proposed method for estimating these compounds was established through chromatographic peak purity analysis. The limit of detection and the limit of quantification were found to be 18.54 µg/mL and 56.20 µg/mL for curcumin, 10.05 µg/mL and 30.46 µg/mL for ascorbic acid, and 11.39 µg/mL and 34.51 µg/mL for salicylic acid respectively. The accuracy of the method was demonstrated by recovering curcumin, ascorbic acid, and salicylic acid from the hydrogel formulation with a recovery rate exceeding 98%. This indicates the capability of the method to accurately estimate active pharmaceutical ingredients in hydrogel dosage form without interference from excipients. Validation results support the potential applicability of the proposed method for the quantitative estimation of these three drugs in hydrogel.
KEYWORDS- High Performance liquid chromatography
- Hydrogel
- Method Development
- Curcumin
- Ascorbic acid
- Salicylic acid