JOURNAL 2962
Journal of Chemical Metrology
Year: 2024 Issue: 1 January-June
p.30 - 40
Viewed 2411 times.
GRAPHICAL ABSTRACT
ABSTRACT
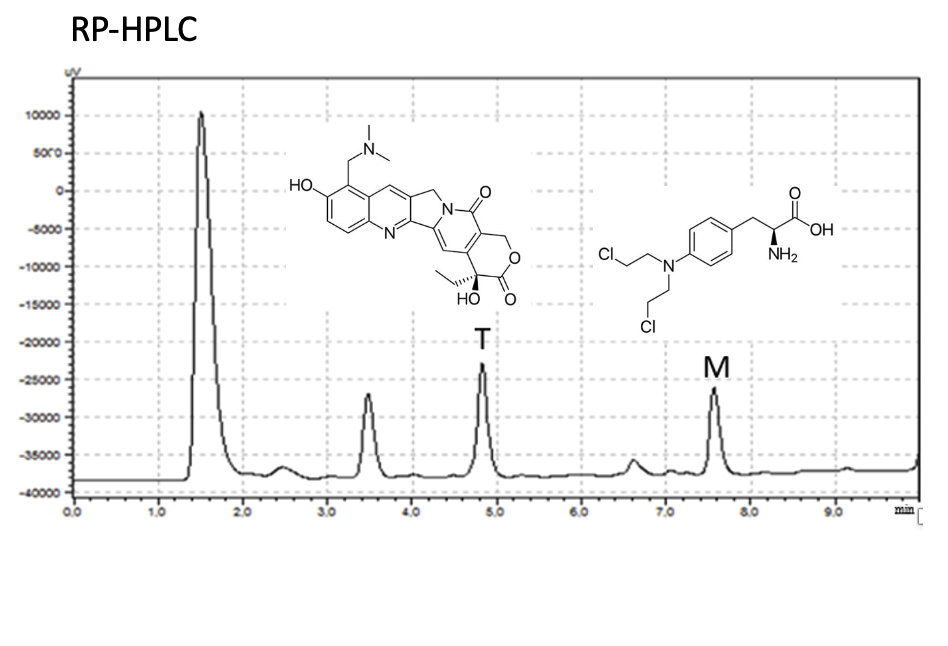
Pharmaceutical analysis still attracts the attention of researchers, and pharmaceutical analysis methods specific to the purpose of the application are needed in routine applications. In this study, the RP-HPLC method was developed for the simultaneous analysis of two different cancer drugs from human blood plasma due to simultaneous use. Melphalan and topotecan are licensed drugs that have been used for a long time. In routine practice, the simultaneous use of these two drugs is limited. However, studies have found that two active substances were used together in high-dose chemotherapy applications. This situation encourages the development of methods for the simultaneous analysis of both active substances in spiked human blood plasma samples. In this study, melphalan and topotecan were analyzed by RP-HPLC in 17 minutes, including post-run, with a gradient elution program using a Superco 5 µm C18 100 Å LC Column (100 x 4.6 mm) when the flow rate was 1.0 mL min-1. The method was linear in the 1.0- 20.0 µg/mL range for both active substances. The detection wavelength was 261 nm. The accuracy and precision of the validated analytical method were demonstrated through intraday and interday studies. The analyte was freed from the remaining plasma components due to filtering the supernatant (after precipitation of the plasma proteins with methanol) with an ultrafilter (having 10 kDa pores). The simple methodology used in this study can be easily adapted to any pre-clinical or clinical application where analysis of melphalan and topotecan in plasma is required.
KEYWORDS- Melphalan
- Topotecan
- RP-HPLC
- pharmaceutical analysis
- ultrafiltration
- validation