JOURNAL 3020
Journal of Chemical Metrology
Year: 2024 Issue: 1 January-June
p.19 - 29
Viewed 2879 times.
GRAPHICAL ABSTRACT
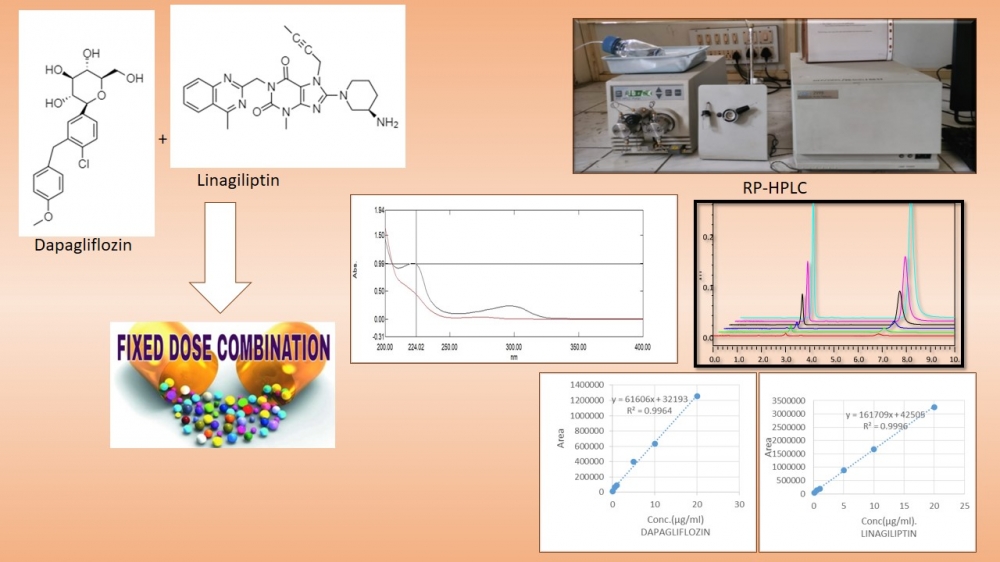
ABSTRACT
Dapagliflozin inhibits selective sodium–glucose co-transporter-2 and Linagliptin competitively and reversibly inhibits dipeptidyl peptidase-4 in fixed dose Combination (1:1) is used in the treatment of Type 2 Diabetes Mellitus. For estimation Dapagliflozin and Linagliptin in bulk and Tablet formulation, an accurate and precise method using RP-HPLC was developed and validated. In the method being discussed here was optimized using Hypersil C18 (250 × 4.6 mm, 5 µm) column as Stationary Phase, Mobile Phase being used is Acetonitrile: Water (90:10) adjusting pH 3 using Ammonium Acetate. The Flow Rate was adjusted to 1ml/min. Both Dapagliflozin and Linagliptin (1:1) sample was detected at analytical wavelength of 244nm using Photo diode array detector. The Linearity Range was between Concentration of 0.1µg/ml to 20µg/ml with correlation Coefficient of 0.995 and 0.999 for Dapagliflozin and Linagiliptin Respectively.
KEYWORDS- Diabetes Mellitus
- dapagliflozin
- linagliptin
- RP-HPLC
- method validation