JOURNAL 1594
Organic Communications
Year: 2020 Issue: 2 April-June
p.51 - 56
Viewed 3654 times.
GRAPHICAL ABSTRACT
ABSTRACT
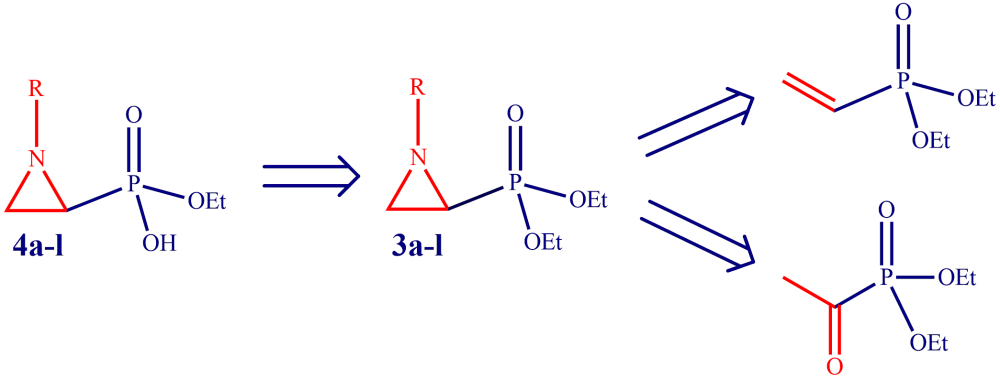
N-substituted aziridine diethyl phosphonates were synthesized easily in two steps starting from vinyl phosphonate or acetyl phosphonate. The controlled mono hydrolysis without opening the aziridine ring under mild reaction conditions was achieved by alkaline solution of LiOH. The corresponding phosphonic acid lithium salt was desalted by the use of Amberlite IRC-50H+ acting as an efficient and recyclable weakly acidic cation exchange promoter. Antimicrobial activity of the synthesized compounds was tested against E. coli ATCC 25922, Staphylococcus aureus (MRSA), Klebsiella pneumoniae NRLL B-4420, Acetobacter baumanii (wild type), Pseudomonas aeroginosa (ATCC 27853) and Enterobacter aerogenes NRLL B-3567. In general, all the compounds showed moderate antibacterial activity.
KEYWORDS- Aziridine-2-phosphonic acids
- atibacterial activity
- hydrolytic cleavage
- ion-exchange