Records of Natural Products
Year: 2019 Volume: 13 Issue:4 July-August
1) A New Diterpene: Abietane Glycoside from the Roots of Isodon rugosus Wall Ex Benth.
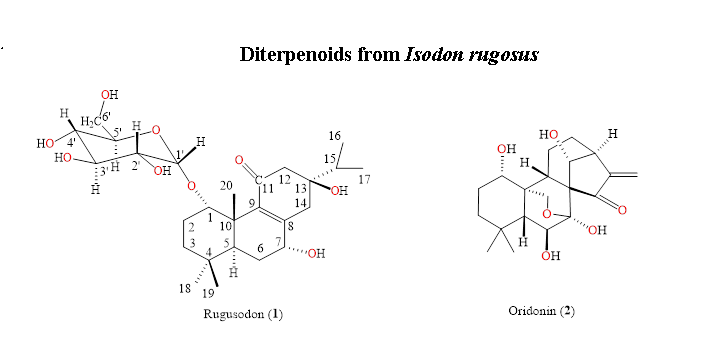
The methanolic extract of the roots of Isodon rugosus were subjected to chromatographic separation, yielded two diterpenes: a new compound named rugosodon 1 and a known compound oridonin 2. The new compound 1 was elucidated as 1-O-α-D-glucopyranosyl-7α,13β-dihydroxyabieta-8(9)-en-11-one and the known compound 2 as 7α,20-epoxy-1α,6β,7,14-tetrahydroxy-kaur-16-en-15-one (oridonin), based on the chemical hydrolysis, physicochemical and spectroscopic data of IR, ESIMS, EIMS, 1D, and 2D NMR. The compounds 1-2 were subjected to bioassay studies of cytotoxicity and α-glucosidase inhibition potential. Rugosodon 1 showed significant potential of α-glucosidase inhibition with IC50 0.453 mg/mL, as compared to the standard acarbose (IC50 0.921 mg/mL). The compounds 1-2 failed to show any significant results for the cytotoxic screening against the three human cancer cell lines (NCI-H460, HeLa and MCF-7).
DOI http://doi.org/10.25135/rnp.101.18.06.310 Keywords Abietane diterpene glycoside Isodon rugosus rugosodon oridonin DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.2) A New Pericarbonyl Lignan from Amauroderma rude

A new pericarbonyl lignan (1), named amaurolignan A was isolated from an ethanol extract of the fruiting bodies in Amauroderma rude of family Ganodermataceae, together with two known lignans, 4-methoxymatairesinol 4′-β-D-glucoside (2) and lappaol F (3). The structures of compounds (1-3) were elucidated using NMR and MS spectroscopic methods.
DOI http://doi.org/10.25135/rnp.115.18.10.1007 Keywords Pericarbonyl lignan amaurolignan A Amauroderma rude DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.3) Phenolic Derivatives from Dioscorea bulbifera
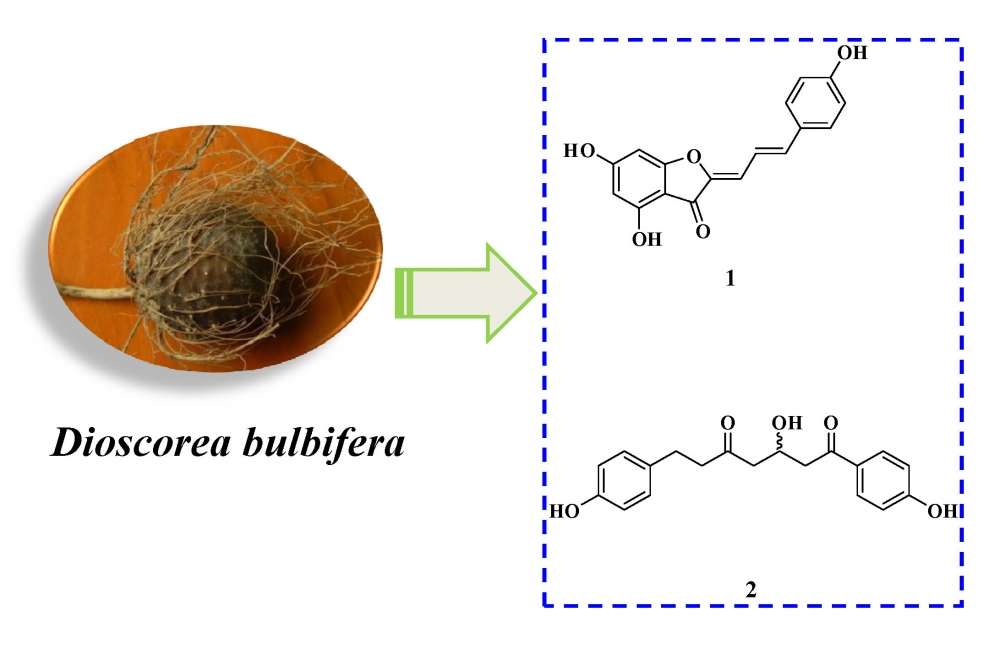
Two new phenolic derivatives, diosbulbiol A (1), diosbulbiol B (2), and six known compounds were isolated from Dioscorea bulbifera. Their structures was determined by MS, IR, UV, 1D- and 2D-NMR. The cytotoxicity of new compounds were evaluated against four cancer cell lines.
DOI http://doi.org/10.25135/rnp.114.18.10.1003 Keywords Dioscorea bulbifera dioscorea cytotoxicity phenolic derivatives diosbulbiol A diosbulbiol B DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.4) Cytotoxicity of Some Plants of the Asteraceae Family: Antiproliferative Activity of Psiadia punctulata Root Sesquiterpenes
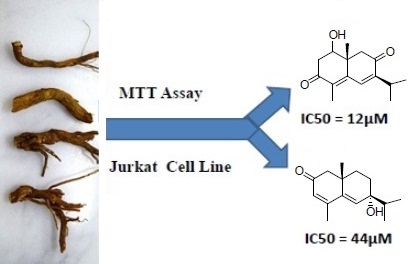
According to the World Health Organization Cancer is the second leading cause of death globally. The methanol extracts of fourteen Middle-Eastern plants of the family Asteraceae were screened for antiproliferative activity on five cancer cell lines (A2780, MCF7, HeLa, RKO and Jurkat) by using MTT assay. Psiadia punctulata (DC.) Vatke was selected for isolation and elucidation of the bioactive constituents by 1D- and 2D-NMR, and MS analyses. Flow cytometry was used to evaluate cell cycle analysis, apoptotic hallmarks and reactive oxygen species. P. punctulata yielded a new sesquiterpene characterized as 7-hydroxy eudesman-3,5-dien-2-one (punctulin) (3) and three known sesquiterpenes: 1, 2 and 4. The antiproliferative activity of all sesquiterpenes was evaluated in Jurkat (T-cell leukemia) and HeLa cancer cell lines. Compound 1 (1β-hydroxy-8-oxo-cyperone) has induced a significant growth inhibition in Jurkat and HeLa cells (IC50 =12 and 18µM respectively). Flow cytometry of compound 1 has elucidated the mechanism of action by showing its ability to induce cell cycle arrest in Jurkat cells mainly in G0/G1 and, less markedly, in G2/M. Compound 1 also expressed strong antioxidant activity by reducing the basal level of peroxides DHFDA-load in Jurkat cells. Compound 1 antioxidant activity may have contributed to the observed cell cycle arrest.
DOI http://doi.org/10.25135/rnp.113.18.10.969 Keywords Cytotoxic Activity Asteraceae Leukemia Cell cycle Sesquiterpenes DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.5) The Chemical Composition of Volatile Oil and Biological Activity of Rhododendron caucasicum
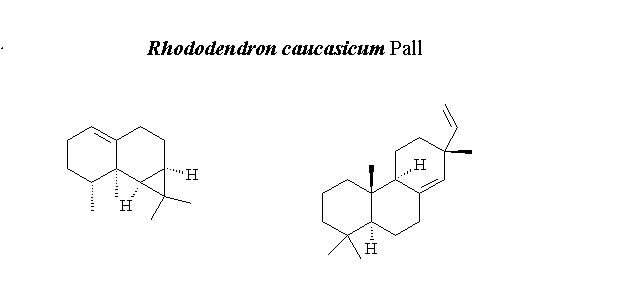
The aim of this research was to investigate the effect of different extraction methods and chemical composition of the essential oil and solid-phase microextraction (SPME) from Rhododendrum caucasium Pall. The volatiles of R. caucasicum have been isolated by hydro distillation (HD) and SPME. The compositions of the volatiles were characterized by GC-FID/MS. A total of twenty-five and thirty-one compounds were identified constituting over 89.25%, and 90.33% of volatiles obtained with HD and SPME, respectively. The main volatile constituents of R. caucasicum were found to be calarene (46.13% (HD) and 54.91% (SPME)) and sandaracopimaradiene (25.93% (HD) and 8.16% (SPME)). Furthermore, the obtained essential oil (EO) and solvent extracts (n-hexane and methanol) of R. caucasicum were tested against the following nine bacteria: Escherichia coli, Yersinia pseudotuberculosis, Pseudomonas aeruginosa, Enterococcus faecalis, Staphylococcus aureus, Bacillus cereus, Mycobacterium smegmatis, Candida albicans, and Saccharomyces cerevisiae. The EO showed moderate antimicrobial activities with the inhibition zone from 6 to18 mm against E. faecalis, S. aureus, B. cereus and M. smegmatis, respectively. Methanol extract gave better antimicrobial activity against the P. aeruginosa, E. faecalis, S. aureus, and B. cereus with the almost 15 mm inhibition zones.
DOI http://doi.org/10.25135/rnp.107.18.09.952 Keywords Rhododendron caucasicum; hydrodistillation; SPME; essential oil; antimicrobial activity; GC-FID/MS. DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.6) Chemical Compositions of Essential Oil of Piper Species from Atlantic Forest of Amazonia, Brazil
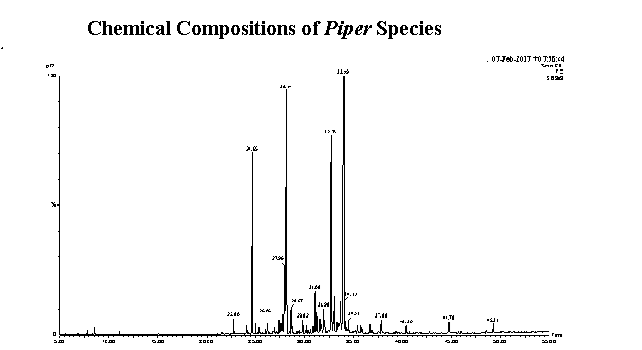
Essential oils from the leaves of Piper japurense (Miq.) C. DC., P. coariense Yunk., P. auriculifolium Yunk., P. curtistilum C.DC., P. alatipetiolatum Yunk. and P. brevesanum Yunk. from the Amazon Forest (Brazil) were obtained through hydrodistillation. The chemical composition of the oils was determined using gas chromatography and mass spectrometry, which revealed the presence of 108 compounds representing 95.14%, 95.64%, 95.57%, 92.05%, 96.24% and 91.316% of the oils, respectively. All oils had an abundance of sesquiterpenes, except the oil from P. alatipetiolatum, which had a higher percentage of monoterpenes. The major components were α-eudesmol in the P. japurense (22.05%) and P. coariense (27.33%) oils, premnaspirodiene (32.26%) in the P. auriculifolium oil, caryophyllene oxide (28.69%) in the P. curtistilum oil, linalool (43.88%) in the P. alatipetiolatum oil and β-elemene (12.75%) in the P. brevesanum oil. Although the oils were composed of terpenes, the chemical analysis revealed qualitative and quantitative differences. This is the first report of the chemical composition of these six species of Piper that occur in the Amazonia biome in Brazil.
DOI http://doi.org/10.25135/rnp.110.18.09.904 Keywords Piper ssp Amazonia biome Essential oil Premnaspirodiene DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.7) Assessment of Endemic Cota fulvida (Asteraceae) for Phytochemical Composition and Inhibitory Activities against Oxidation, alpha-Amylase, Lipoxygenase, Xanthine Oxidase and Tyrosinase Enzymes
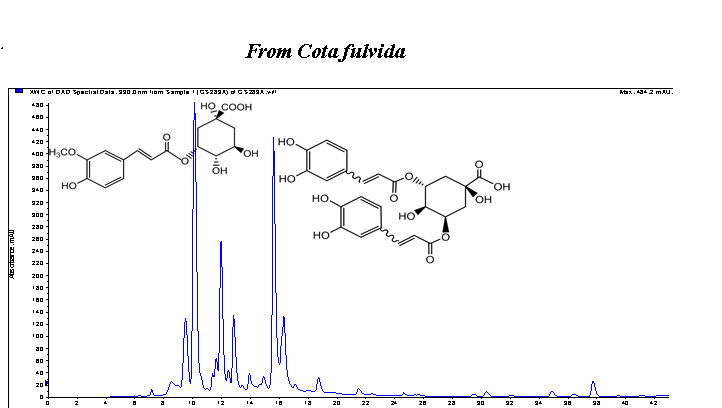
In the present work, chemical compositions of essential oil and methanol extract of endemic Cota fulvida (Grierson) Holub were investigated as well as their antioxidant, antidiabetic, antiinflammatory and antimelanogenesis potent. The phytochemical analyses have been performed with GC-MS/FID and LC-MS/MS techniques. The essential oil was characterized with hexadecanoic acid (25.6 %), camphor (6.1 %), caryophyllene oxide (5.3 %), 1,8-cineole (4.9 %) and humulene epoxide (3.9 %). In the extract, phenolic acids, phenylpropanoid dimer and flavonoids were detected. The major constituents of the extracts were found to be 5-feruloylquinic acid, caftaric acid, 3,5-O-dicafeoylquinic acid and quercetin rutinoside. The antioxidant activities of the oil and extract were evaluated through scavenging of free radicals, inhibition of linoleic acid peroxidation and superoxide anion radical (O2-) generated by xanthine - xanthine oxidase (XO) system. The extract showed free radical scavenging activity (IC50 0.131 mg/mL), Trolox equivalent antioxidant capacity (1.33 mM) and inhibited (Inh. 36.3 %) peroxidation of lipids. The oil and extract demonstrated significant hypoglycemic activity via inhibition of porcine pancreatic a-amylase. The antiinflammatory effects of the oil and extract via inhibition of 5-LOX enzyme were found as 53.7 % and 23.9 %, respectively. The extract demonstrated moderate inhibitory effect (23.3 %) on oxidation of L-DOPA via inhibition of tyrosinase enzyme.
DOI http://doi.org/10.25135/rnp.109.18.09.875 Keywords Cota fulvida Essential Oil Extract GC-MS/FID LC-MS/MS Antioxidant DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.8) Chemical Composition and Biological Activities of the Essential Oil from Aristolochia fordiana Hemsl
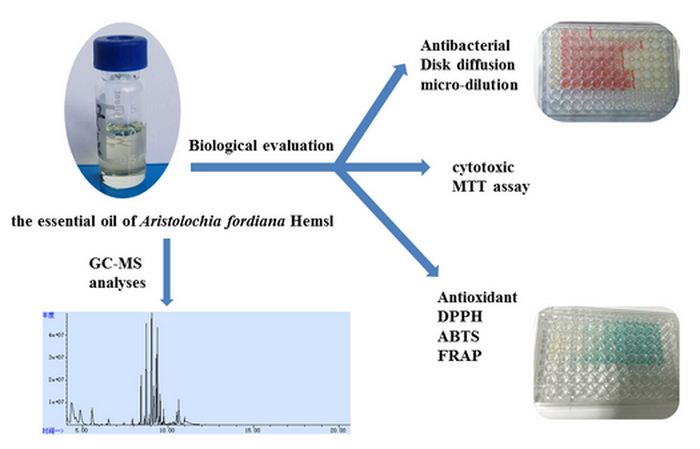
Abstract: The present study investigated the chemical composition of the essential oil obtained from the aerial parts of Aristolochia fordiana Hemsl (AF-EO) using GC-FID and GC-MS, and evaluated the in vitro biological activities of the essential oil. Forty-nine compounds representing 99.6% of the total oil were characterized. The main constituents were identified as β-chamigrene (17.0%), β-caryophyllene (11.1%), α-bulnesene (11.0%) and β-pinene (10.2%). Furthermore, the antibacterial activity of the essential oil of A. fordiana was studied using disc diffusion and micro-broth dilution assays. AF-EO exhibited a significant antibacterial activity against Staphylococcus aureus and Bacillus subtilis with MIC values below 100 μg/mL. Besides, the results of MTT assays indicated that the essential oil exhibited a moderate cytotoxic activity on HepG2 (liver hepatocellular cells) and MCF-7 (human breast adenocarcinoma cells) cell lines. However, the AF-EO showed a weak antioxidant activity in DPPH•, ABTS•+ and FRAP assays.
DOI http://doi.org/10.25135/rnp.111.18.09.897 Keywords Aristolochia fordiana Hemsl essential oil antibacterial activity cytotoxic activity antioxidant activity DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.9) Chemical Composition, Enantiomeric Distribution and AChE-BChE Activities of the Essential Oil of Myrteola phylicoides (Benth) Landrum from Ecuador
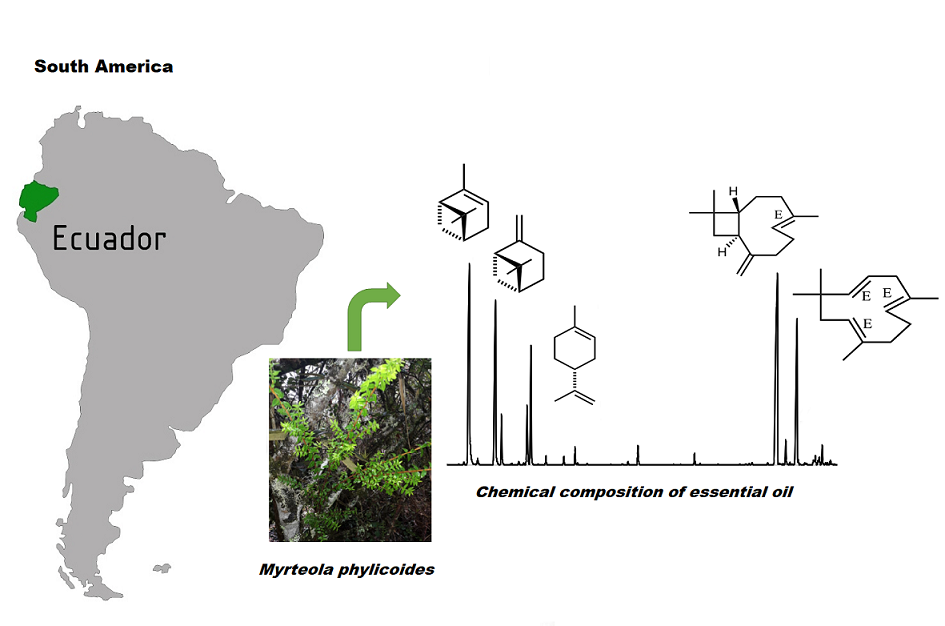
The volatile constituents of the essential oil (EO) of Myrteola phylicoides (Benth) Landrum, from Ecuador, extracted by steam distillation have been analyzed. A total of 37 compounds, representing 90.30% the total essential oil sample were identified. Monoterpenes hydrocarbons (53.06%) and sesquiterpene hydrocarbons (35.24%) were the principal groups of compounds. The major components were identified as α-pinene (30.94%), (E)-caryophyllene (21.93%), β-pinene (14.45%) and α-humulene (9.56%). The essential oil of M. phylicoides showed weak in vitro activity against AChE inhibition with IC50 value 60.8 μg/mL and a low BChE activity with IC50 value >250 μg/mL. This is the first report on the chemical composition of the essential oil of this species.
DOI http://doi.org/10.25135/rnp.112.18.09.893 Keywords Myrteola phylicoides essential oil AChE BChE DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.10) Glutinosine A: A New Morphinandienone Alkaloid from Litsea glutinosa
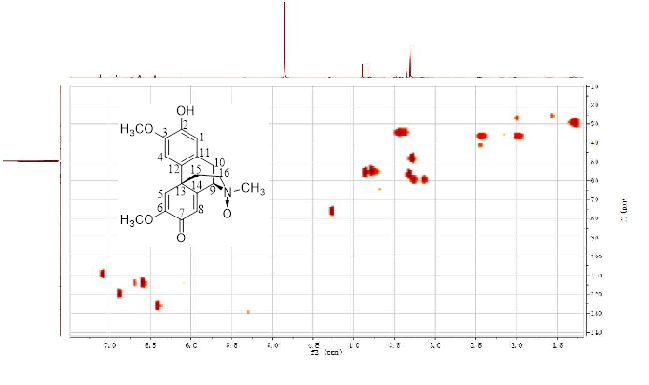
A new morphinandienone alkaloid, named glutinosine A was isolated from the root barks of Litsea glutinosa. The new structure was determined by various spectroscopic techniques including 1D (1H-, 13C-NMR), 2D-NMR (HMBC, HSQC, COSY and ROESY) and high resolution electrospray ionization mass spectrometry (HR-ESI-MS). The effects of the new compound on glucose consumption in HepG2 cells were evaluated. Whereas, the result showed that this compound displayed no activity in stimulating glucose consumption
DOI http://doi.org/10.25135/rnp.92.18.06.306 Keywords Litsea glutinosa Glutinosine A morphinandienone glucose consumption activities DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.