JOURNAL 2545
Journal of Chemical Metrology
Year: 2022 Issue: 2 July-December
p.111 - 124
Viewed 2898 times.
GRAPHICAL ABSTRACT
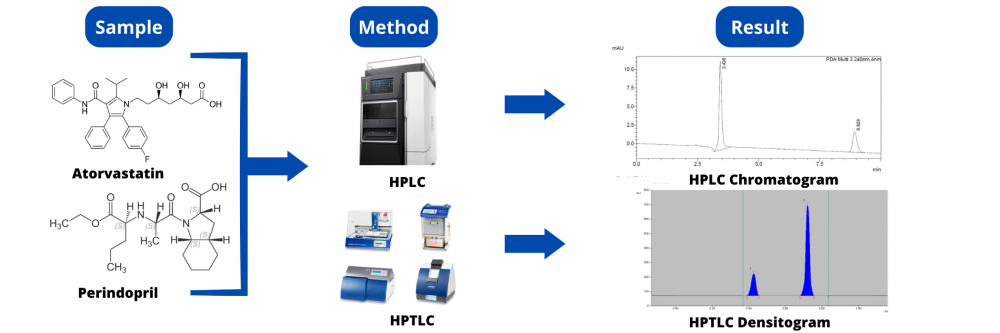
ABSTRACT
Two simple, selective, precise, and accurate chromatographic methods, namely reversed-phase high-performance liquid chromatography (RP-HPLC) method and High-performance thin-layer chromatography (HPTLC), were developed and validated for the simultaneous determination of Atorvastatin (ATO) and Perindopril (PER). The developed HPTLC method was used for the separation and quantitation of the studied drugs on silica gel 60F254 TLC plates. Dichloromethane: Methanol: Ethyl acetate: Glacial acetic acid (6:2:2:0.1, v/v/v/v) was used as a developing system and the separated bands were UV-scanned at 221 nm. Linear relationships were obtained in the range of 800 – 4800 ng/band for Atorvastatin and 200 – 1200 ng/band for Perindopril with a regression coefficient greater than 0.999. The Rf value of the drug was found to be 0.70 ± 0.02 and 0.39 ± 0.03 for Atorvastatin and Perindopril respectively. The developed RP-HPLC depended on chromatographic separation of the studied drugs on a C18 column using Acetonitrile: Methanol: Potassium dihydrogen orthophosphate (pH 3 adjusted with orthophosphoric acid) ((40:10:50), v/v/v) as a mobile phase delivered at a constant flow rate of 1 mL/min with UV detection at 240 nm. The calibration curves were linear (r2 > 0.999) over the concentration range 20-100 µg/mL for Atorvastatin and 10-50µg/mL for Perindopril. The average retention times for Atorvastatin and Perindopril were 3.42 and 8.92 min, respectively. Factors affecting the developed methods have been studied and optimized. Further, methods validation has been carried out according to ICH guidelines. The proposed methods were successfully applied for the determination of the studied drug simultaneously in bulk and synthetic mixture qualitatively and quantitatively. Statistical analysis by the F test showed no significant difference between the results obtained by the two methods. The uncertainty measurement was also carried out for the quantification of both components. The proposed HPTLC method proved to be more sensitive, while the HPLC gave more reproducible results and was time-saving.
KEYWORDS- Atorvastatin
- perindopril
- HPLC Method
- HPTLC Method
- statistical comparison
- Method Validation