JOURNAL 2636
Journal of Chemical Metrology
Year: 2022 Issue: 2 July-December
p.125 - 134
Viewed 2663 times.
GRAPHICAL ABSTRACT
ABSTRACT
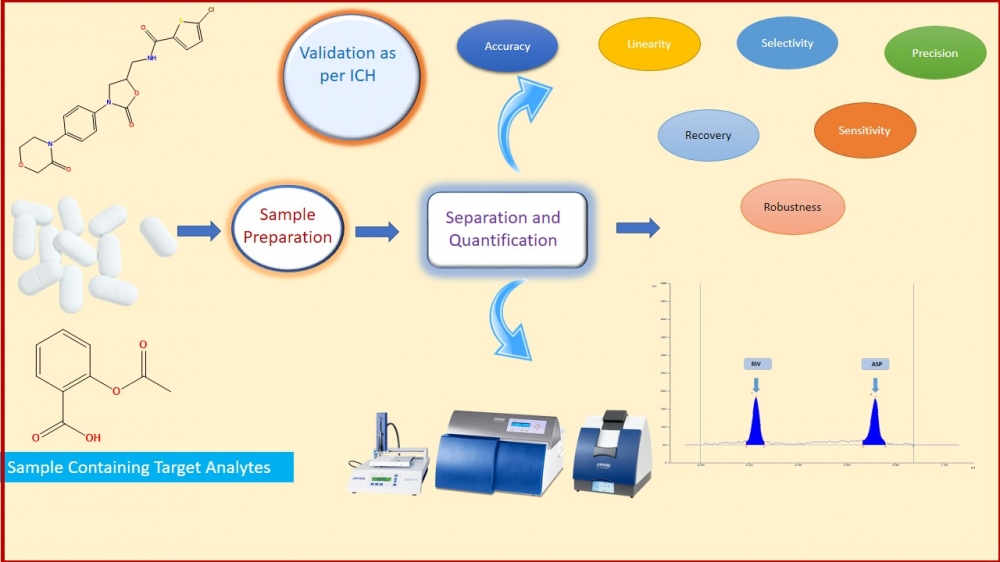
In the present work, simple, precise, accurate high-performance thin-layer chromatography was developed, optimized, and validated for quantitation of Rivaroxaban (RIV) and Aspirin (ASP). The developed method was applied for quantification of both the drugs simultaneously in bulk drug and in-house tablet formulation. In this study, the Camag Linomat V HPTLC system and win CATS software V1.4.7 were used. Both molecules were separated using a chromatographic method consisting of toluene: ethyl acetate: methanol: glacial acetic acid (6:3:0.5:0.5 v/v/v/v) as mobile phase & an aluminum pre-coated plate with silica gel 60 F254 as the stationary phase. Both drugs were detected at 256nm. With Rf values of 0.23 and 0.72, respectively, Rivaroxaban and aspirin were satisfactorily resolved. Moreover, as per the ICHQ2 (R1) guideline, Specificity, precision, accuracy, robustness, linearity, the limit of detection, and the limit of quantification were performed. The method was found linear in the range of 100-400 ng/band and 2000-8000 ng/band of Rivaroxaban and Aspirin, respectively. The precision of the method was determined by %RSD and it was found in range. In-house tablet formulation was prepared and applied the developed method for assay of RIV and ASP. The % Assay (%v/v) of RIV and ASP was found 100.61 %w/w and 100.29 %w/w. In a conclusion, the accurate, precise, sensitive, and robust HPTLC method was optimized, developed, and validated as per ICH Q2 (R1) guideline, which was applied for in-house tablet formulation. The result of the assay suggests that the developed method can be used for simultaneous estimation of RIV and ASP for their dosage form as a part of regulatory submission.
KEYWORDS- Rivaroxaban
- high performance thin layer chromatography
- validation
- ICH guidelines
- tablet formulation.