JOURNAL 1323
Organic Communications
VOLUME & ISSUE
Year: 2019 Issue: 3 July-September
Year: 2019 Issue: 3 July-September
PAGES
p.121 - 131
p.121 - 131
STATISTICS
Viewed 3487 times.
Viewed 3487 times.
GRAPHICAL ABSTRACT
ABSTRACT
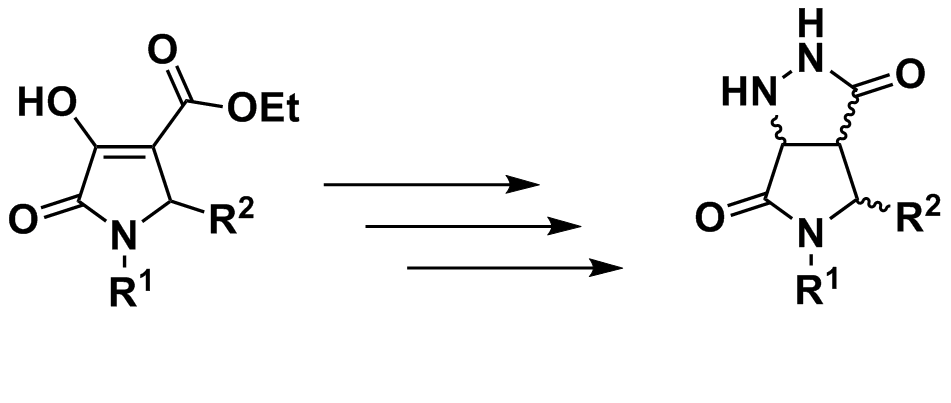
A new pathway for the synthesis of novel 3,4-fused pyrazolidinone-γ-lactam (4) starting from 2,3-dioxo-4-carboxy-5-(substituted)pyrrolidines (1) was developed. The key synthetic strategy involved hydrazonation of the precursor 1 followed by subsequent reduction reaction of the enamino ester 2 and intramolecular cyclization reaction to furnish the unprecedented 3,4-fused pyrazolidinone-γ- lactam (4) in reasonable yield. The structures of all synthesized compounds were confirmed by spectroscopic methods and chemical transformation.
KEYWORDS- Pyrazolidinone-γ-lactam
- Pyrazolidinone
- Pyrrolidinone
- Bicyclic γ-lactam